1839
Accuracy and test-retest repeatability of stiffness measurement with MR Elastography: a multi-center phantom study
Efe Ozkaya1,2, Paul Kennedy1,2, Jun Chen3, Octavia Bane1,2, Jonathan R. Dillman4, Kartik S. Jhaveri5, Michael Ohliger 6,7, Phillip J. Rossman3, Jean A. Tkach4, Sudhakar K. Venkatesh3, Richard L. Ehman3, and Bachir Taouli1,2
1Department of Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Department of Radiology, Mayo Clinic, Rochester, MN, United States, 4Department of Radiology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, United States, 5Joint Department of Medical Imaging, University Health Network, Mount Sinai Hospital, and Women’s College Hospital,, University of Toronto, Toronto, ON, Canada, 6Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 7Department of Radiology, Zuckerberg San Francisco General Hospital, San Francisco, CA, United States
1Department of Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Department of Radiology, Mayo Clinic, Rochester, MN, United States, 4Department of Radiology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, United States, 5Joint Department of Medical Imaging, University Health Network, Mount Sinai Hospital, and Women’s College Hospital,, University of Toronto, Toronto, ON, Canada, 6Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 7Department of Radiology, Zuckerberg San Francisco General Hospital, San Francisco, CA, United States
Synopsis
Keywords: Liver, Elastography, Phantom, Multicenter study, Accuracy, Repeatability
The purpose of our multicenter study was to determine the accuracy and test-retest repeatability of stiffness measured with MRE in phantoms. Three phantoms with known stiffness (2.4, 4.4, and 7.5 kPa) were circulated between 5 different centers. 1.5T or 3T systems from the 3 major vendors were used for scanning with 2D GRE and/or SE-EPI MRE sequences. The reference was based on measurements in the reference center made at the start and end of study. For all 3 phantoms the mean accuracy error for the 4 testing centers was 10.2%, while test-retest repeatability error was 2.9% for all centers.Introduction
MRE is gaining clinical acceptance due to its high performance for noninvasive staging of liver fibrosis [1,2,12]. There is however a need to assess the measurement variability between systems and vendors, for the purpose of multicenter research studies and drug trials. There are a few reports on the test-retest repeatability and inter-platform reproducibility of MRE acquired stiffness measurements [3-11], however; there is a lack of data on inter-platform variability assessed in a multi-center study design.Methods
As this prospective in-vitro study did not involve human subjects or animals, IRB approval was not necessary. Three PVC phantoms were constructed at the reference center (center 1), mixing plastic and plastic softener in the following percentage mixing ratios: 44-55 (phantom I, low stiffness: 2.4 kPa), 55-45 (phantom II, medium stiffness: 4.4 kPa), and 65-35 (phantom III, high stiffness: 7.5 kPa). The phantoms used in the study were shipped from the reference center to all test centers (centers 2-5). Reference measurements were collected at the start (12/16/2019) and end of the study (12/07/2021), to account for changes in stiffness with time. Center 2 served as the coordinating center and performed all data analysis. MRE measurements were obtained on 10 different scanner systems at 1.5T (5 scanners) and 3T (5 scanners) using 3 main vendors (1-3) among which the 2D SE-EPI MRE sequence was used on 6, while 2D GRE MRE was used on all. Sequence parameters are provided in Table 1 and center-specific scanner systems with the MRE sequences available are given in Table 2. For test-retest repeatability, a 2nd measurement under the same conditions was performed in all centers. A single observer (with 5 years of experience) analyzed the data, by using a mask of 10.8 cm in diameter on each acquired elastogram to measure mean stiffness (kPa). Calculation of measurement accuracy error was done for all testing centers with respect to the average of the initial and last MRE measurement done at the reference center. Test-retest repeatability error was calculated as the percentage difference of stiffness values measured in duplicate relative to the mean of two measured values for all centers.Results
The ground truth stiffness values were as follows (average measurement in reference center using GRE sequence on the same 1.5T system using vendor 2): 2.4 kPa for phantom I, 4.4 kPa for phantom II, and 7.5 kPa for phantom III as depicted in Figure 1. Mean accuracy error from 66 measurements including 4 testing centers, 3 phantoms and both sequences was 10.2%. Mean accuracy error was found to be dependent on the phantom being assessed: phantom I: 8.3%, phantom II: 8.8%, and highest for phantom III: 13.1% (Figure 2a). The test-retest repeatability error was 2.9% for all types of phantoms combined, with 3.0%, 1.9%, and 3.7% for phantoms I, II, and III respectively (Figure 2b).Discussion
The importance of this in vitro work relies on the fact that inter-platform measurement of stiffness was done on 3 different phantoms that represent a range of stiffness values similar to that found in human liver fibrosis [1,2,12]. In the study done by Yasar et al, only the 2D GRE sequence was used for MRE acquisition on two separate vendor platforms. They reported an excellent interplatform reproducibility for a single phantom being tested with coefficient of variability of 4.4% [4]. Trout et al. inspected the MRE-acquired liver stiffness repeatability and agreement through a wide variety of MR imaging systems under different field strengths and pulse sequence combinations with a conclusion of MRE is a reliable method for liver stiffness assessment with coefficient of variation of 10.7% [7]. Our study had few limitations. First, we just measured the stiffness of a homogenous phantoms. Second, our ground truth measurements of phantom stiffness were obtained from MRE. To address these limitations, in the future, stiffness measurements can be performed on phantoms having inclusions with varying degrees of stiffness and ground truth stiffness measurements can be done on rheometry measuring system. Finally, phantom measurements may not necessarily reflect in-vivo conditions, which may have additional impact on reproducibility of stiffness measurement.Conclusion
The overall accuracy of MRE observed in this multi-center study with a phantom mimicking human liver stiffness values was excellent, slightly lower for higher stiffness values. Test-retest repeatability was excellent.Acknowledgements
No acknowledgement found.References
- Venkatesh, Sudhakar K., Meng Yin, and Richard L. Ehman. "Magnetic resonance elastography of liver: technique, analysis, and clinical applications." Journal of magnetic resonance imaging 37.3 (2013): 544-555.2.
- Venkatesh, Sudhakar Kundapur, and Richard L. Ehman. "Magnetic resonance elastography of liver." Magnetic Resonance Imaging Clinics 22.3 (2014): 433-446.3.
- Serai, Suraj D., et al. "Cross-vendor validation of liver magnetic resonance elastography." Abdominal imaging 40.4 (2015): 789-794.4.
- Yasar, Temel Kaya, et al. "Interplatform reproducibility of liver and spleen stiffness measured with MR elastography." Journal of Magnetic Resonance Imaging 43.5 (2016): 1064-1072.5.
- Wang, Kang, et al. "Repeatability and reproducibility of 2D and 3D hepatic MR elastography with rigid and flexible drivers at end-expiration and end-inspiration in healthy volunteers." Abdominal Radiology 42.12 (2017): 2843-2854.6.
- Kim, Hye Jin, et al. "Reproducibility of hepatic MR elastography across field strengths, pulse sequences, scan intervals, and readers." Abdominal Radiology 45.1 (2020): 107-115.7.
- Trout, Andrew T., et al. "Liver stiffness measurements with MR elastography: agreement and repeatability across imaging systems, field strengths, and pulse sequences." Radiology 281.3 (2016): 793-804.8.
- Sawh, Mary Catherine, et al. "Normal range for MR elastography measured liver stiffness in children without liver disease." Journal of Magnetic Resonance Imaging 51.3 (2020): 919-927.9.
- Serai, Suraj D., et al. "Repeatability of MR elastography of liver: a meta-analysis." Radiology 285.1 (2017): 92.10.
- Kim, Yong Seek, et al. "Comparison of spin-echo echoplanar imaging and gradient recalled echo-based MR elastography at 3 Tesla with and without gadoxetic acid administration." European Radiology 27.10 (2017): 4120-4128.11.
- Calle-Toro, Juan S., et al. "Magnetic resonance elastography SE-EPI vs GRE sequences at 3T in a pediatric population with liver disease." Abdominal Radiology 44.3 (2019): 894-902.12.
- Kennedy, Paul, et al. "Quantitative elastography methods in liver disease: current evidence and future directions." Radiology 286.3 (2018): 738.
Figures
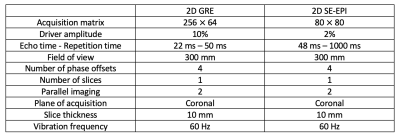
Table 1: MRE sequence parameters of 2D GRE and 2D SE-EPI
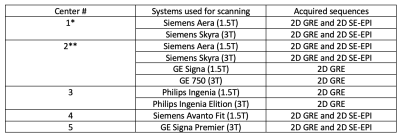
Table 2: List of centers, MRI scanners, and imaging sequences (*Reference center,**Coordinating center). Test centers; centers 2-5, reference center; center 1.
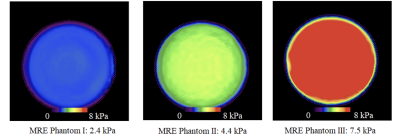
Figure 1: The ground truth stiffness maps of the three different phantoms constructed by the reference center.
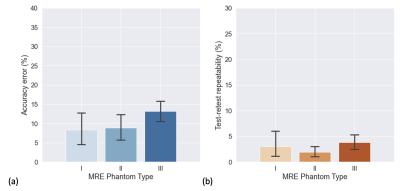
Figure 2: Phantom specific (a) mean accuracy error calculated for 4 testing centers and (b) test-retest repeatability error calculated for 5 centers (including the testing and reference center).
DOI: https://doi.org/10.58530/2023/1839