1835
Multi-Echo MRI Motion Artifact Reduction via Knowledge Interaction Learning for Better SWI Enhancement1Department of Artificial Intelligence, Sejong University, Seoul, Korea, Republic of, 2Department of Electrical and Electronic Engineering, Yonsei University, Seoul, Korea, Republic of, 3GE Healthcare, Korea, Seoul, Korea, Republic of, 4Department of Radiology, Seoul National University Hospital, Seoul, Korea, Republic of
Synopsis
Keywords: Motion Correction, Artifacts
Patient movement during MRI scan can cause severe degradation of image quality. In Susceptibility-Weighted Imaging (SWI), several echoes are measured during a single repetition period, where the earliest echoes show less contrast between various tissues, while the higher echoes are more susceptible to artifacts and signal dropout. This paper proposes a data-driven retrospective deep learning method by taking the advantage of interactively learning multiple echoes together through sharing their knowledge using unified training parameters. The proposed method allows to share information and gain an understanding of the correlations between multiple echoes towards generating high-resolution susceptibility enhanced contrast images.Introduction
Any movement with even a few millimeters during MRI scanning can produce motion artefact, which may result in unacceptable diagnostic exams for medical analysis 1. The multi-echo readout method has the ability to integrate a wide range of various tissue contrasts with improved susceptibility information 2. Thus, all TE readouts are exposed to degradation if motion occurred during the scanning procedure, and the artifact gets accumulated at the time of reconstructing Susceptibility-Weighted Imaging (SWI) that compiles all the measured echoes. In this study, we propose a data-driven retrospective deep learning method by taking the advantage of interactively learning multiple echoes together through sharing their knowledge using unified training parameters.Methods
[Dataset]In this study, we collected total of 138 motion-free clinical subjects scanned by a 3.0 Tesla MRI GE scanner. The protocol for all collected data acquired four echo readouts per subject with TE=10.0, 18.17, 26.35, and 34.53 ms within a TR=50 ms. Scanning different pairs of reference motion-free and distorted motion-corrupted data is challenging. We supposed that the rigid bulk motion is a combination of 3D rotation and translation motions. This study performs the motion artifacts simulation on two domains: image-based and k-space-based simulations. More details about motion artifacts simulation exist in our published works 3,4.
[Proposed Network]
The primary goal of the proposed deep learning motion artifacts correction network is to retrieve high-quality motion-free images from the distorted motion-corrupted data by enabling the network to learn optimal relationships among multiple echoes themselves and to map them to the corresponding clean data in a supervised learning paradigm. This paper develops a Knowledge Interaction Learning approach that shares information and gains an understanding of the correlations between Multiple Echoes (KIL-ME) for better motion artifacts correction of all distorted echoes simultaneously. This work develops a new scheme for the incorporation of multi-echo inputs using a Single Encoder and Multiple Decoders, called KIL-ME-based SEMD (see Figure 1). The proposed KIL-ME-based SEMD method has the ability to fuse and learn the extracted features from all TE inputs using a single encoder. The encoder interactively enables learning and sharing knowledge of structural details and artifacts between multi-echo inputs in a robust way utilizing unified training parameters. Since the brain structure has very high similarity in all measured echoes, this might assist in retrieving the missed structure details due to motion artifacts during jointly fusing the learned features. The proposed method is eventually able to reconstruct an enhanced SWI from corrupted TE images, increasing the readers’ capability to accurately diagnose MRI brain data.
Results
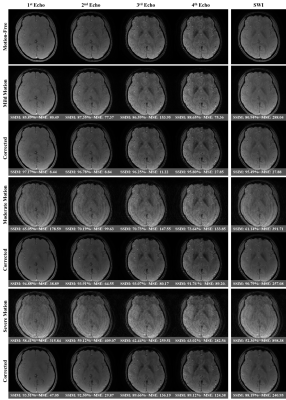
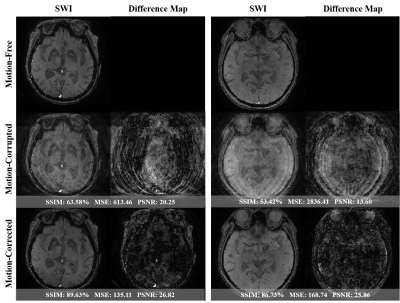
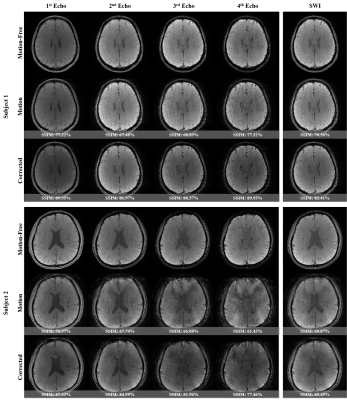
We investigated four different potential solutions to tackle the mitigation of motion artifacts using multi-echo data. Note that ‘EXP0’ denotes the simulated motion-corrupted data, ‘EXP1’ refers to the separate treatment of each echo data, ‘EXP2’ shows a solution of merging all the acquired TEs to build a larger training pool, ‘EXP3’ presents a multi-input multi-output scheme with a feature fusion module called MEMD-FFM, and lastly ‘EXP4’ shows our proposed method via a single learnable encoder named KIL-ME-based SEMD. It is noteworthy that the generated SWI map in ‘EXP1’ requires multiple training and testing for each echo data. Both ‘EXP1’ and ‘EXP2’ approaches disregard the benefits of sharing knowledge within multi-echo data. The performance of all these potential solutions is summarized in Figure 2. The results showed that the proposed approach outperformed the other methods with SSIM, MSE, and PSNR improvement rates of 17.92%, 83.58%, and 24.23% compared to the simulated motion-corrupted data, respectively. Figure 3 qualitatively shows the motion-corrected results of the proposed method over different motion severities of the same motion-corrupted samples. Figure 4 shows exemplary results of the corrected SWI maps from two different subjects with moderate and severe motions. The results revealed the capability of the proposed method to efficiently reduce motion artifacts and enhance the image quality, particularly the vessel structural details. Figure 5 shows the results of two real volunteer cases for all acquired TEs and their relevant SWI maps. Despite of that our proposed network was trained on simulated motions, the reconstructed motion-corrected images via the proposed method succeeded to some extent in reducing the artifacts from real distorted motion data.Discussion
The proposed method successfully recovered high structural details from distorted images, especially when there are only slight motions. This is because the proposed approach is effective at transferring knowledge from various echo data, in which the network enables interactively learning additional contrast details and motion patterns, and hence improve the correction performance. Even though the contrast between different tissues and the signal intensity strengths in the TE readouts varies, the motion-corrected results for all echoes were promising for all presented samples. The reconstructed images for early echoes provided high structural similarity compared to the reference motion-free images (i.e., SSIM > 0.93), while the late TEs presented a better contrast between tissues.Conclusion
The proposed method can successfully generate an enhanced SWI map from motion-corrupted TEs, improving the readers’ capability to precisely diagnose MRI brain data. The findings from both motion-simulated testing data and actual volunteer data demonstrate the feasibility and effectiveness of the proposed method in reducing motion artifacts and improving the overall clinical image quality.Acknowledgements
This work was supported in part by GE Healthcare research funds and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1A2C1090635).References
1 Wood, M. L. & Henkelman, R. M. MR Image Artifacts from Periodic Motion. Med Phys 12, 143-151, doi:10.1118/1.595782 (1985).
2 Kovářová, A., Gajdoš, M., Rektor, I., & Mikl, M. Contribution of the multi‐echo approach in accelerated functional magnetic resonance imaging multiband acquisition. Human brain mapping 43(3), 955-973 (2022).
3 Al-Masni, M. A. et al. Stacked U-Nets with Self-Assisted Priors Towards Robust Correction of Rigid Motion Artifact in Brain MRI. Neuroimage 259, 119411, doi:10.1016/j.neuroimage.2022.119411 (2022).
4 Lee, S., Jung, S., Jung, K.-J. & Kim, D.-H. Deep Learning in MR Motion Correction: a Brief Review and a New Motion Simulation Tool (view2Dmotion). Investigative Magnetic Resonance Imaging 24, 196-206 (2020).
Figures