1816
Amide proton transfer-weighted imaging for hepatocellular carcinoma: Correlation with Ki-67 labeling index1Clinical Medical School of Yangzhou University, Northern Jiangsu People’s Hospital, Yangzhou, China, Yangzhou City, China, 2GE Healthcare, MR Research China, Beijing, P.R. China, Beijing City, China
Synopsis
Keywords: Quantitative Imaging, CEST & MT, APTw
Conventional MRI methods are difficult to reveal histologic and molecular characteristic heterogeneity of HCC. In this study, we aimed to investigate the relationship between APTw and Ki-67 labeling index (LI) in hepatocellular carcinoma (HCC) to evaluate whether APTw can reflect the pathological information of HCC at the molecular level. It was found that APTw values were significantly different between low and high Ki-67 LI in HCC, and the AUC to distinguish high and low Ki-67 LI was 0.794, indicating APTw imaging might be a potential molecular technique for predicting the malignancy grade of HCCIntroduction
Hepatocellular carcinoma is one of the leading causes of cancer-related mortality worldwide. Ki-67, a protein associated with cellular proliferation, can predict the malignant potential of tumors[1]. Recently, a meta-analysis showed that high Ki-67 revealed the potential deterioration of HCC, as presented by large tumor size, multiple tumor nodes, tumor metastasis, cirrhosis, and vein invasion[2]. Hence, it was suggested that Ki-67 might be a biomarker for clinical deterioration and poor prognosis in HCC. Amide proton transfer weighted (APTw) imaging focuses on proton exchange between amide protons of peptides and proteins and bulk water[3,4]. APTw imaging has been extensively applied for predicting the Ki-67 proliferation status of tumors, including meningioma, endometrial carcinoma and rectal adenocarcinoma[5-7]. Moreover, two recent studies showed the potential of APTw imaging in predicting the histologic grades of HCC. Thus, we hypothesized that APTw imaging may be helpful to predict the Ki-67 expression in hepatocellular carcinoma. Therefore, the purpose of this study was to explore the feasibility of APTw imaging in predicting the Ki-67 LI of hepatocellular carcinoma.Materials and Methods
SubjectsThe study was approved by the local ethical community, and consent forms were obtained from all patients. 8 patients were categorized into the low Ki-67 LI group (≤ 10%) and 24 patients were categorized into the high Ki-67 LI group (> 10%). All patients received liver tumor resection and pathological examination.
MRI experiments
All patients underwent liver MR scanning on a 3.0-tesla scanner (GE DISCOVERY MR750; Milwaukee, Wisconsin, USA) with a 32-channel phased-array torso coil. Before the examination, all patients underwent fasting for 4-6 hours. Routine liver tumor scanning protocol was used, including T2-weighted imaging, T1-weighted imaging, and diffusion-weighted imaging. Before contrast injection, APTw imaging was performed with a respiratory triggered single slice spin-echo echo-planar-imaging sequence. Images at 52 frequencies were acquired, including 49 frequencies ranging from -600 to 600 Hz with an increment of 25 Hz and 3 unsaturated images (M0). The applied saturation power was 2µT and the saturation duration was 2000ms. Other scan parameters were:TE=32.7ms, TR=5432ms, FOV=34cm × 26 cm, Matrix size=128 × 128, and slice thickness=8mm. Scan time was around 2 minutes and 52 seconds.
Imaging analysis
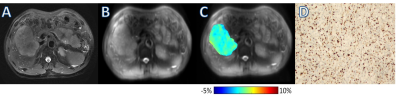
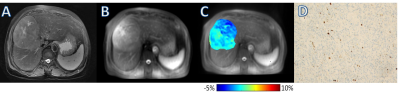
Magnetization transfer ratio asymmetry (MTRasym) image at 3.5ppm was obtained for each patient. Two radiologists with 5 and 13 experiences were employed for data analysis. With the reference of axial T2WI images, three circular regions of interest (ROIs) with approximately 40-50mm2 were placed manually in the solid component of the tumor for each patient on unsaturated M0 images. Large cystic cavities, large areas of necrosis, calcification, hemorrhage, and large vessels were excluded from ROI selections. ROIs of tumors were copied on the MTRasym map. Then the average APTw in the three ROIs for each radiologist was obtained for subsequent analyses.
Statistical analysis
All statistical analyses were performed in SPSS 23.0. The inter-class correction coefficient (ICC) was used to evaluate the inter-observer agreement of measuring the APTw value between two radiologists. ICC>0.75 was considered good reproducibility. The comparisons between APTw values for low and high Ki-67 LI groups were analyzed using the independent t test. Receiver operating characteristic (ROC) curves were generated for MTRasym to obtain the areas under the curve (AUC). Pearson's correlation analysis was used to investigate the association between Ki-67 LI and APTw value.
Results
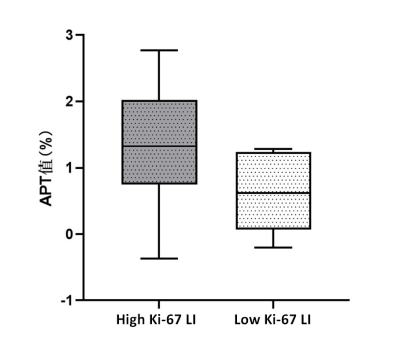
Ki-67 LI of the enrolled patients ranged from 1% to 90%. The ICC of the two observers’ measurements for the APTw value was 0.893. Pearson’s correlation analysis showed that APTw value was positively correlated with Ki-67 LI (r=0.377, P=0.034). The APTw value of the high Ki-67 LI group was significantly higher than that of the low Ki-67 LI group [ (1.33±0.77) % vs (0.64±0.58) %; P=0.026] (Fig. 1). The cut-off APTw value for differentiating low- and high-proliferation groups was 1.24% (sensitivity, 67%; specificity, 88%; AUC, 0.794, 95%CI: 0.615, 0.916). Representative cases are shown in Fig. 2 and Fig. 3.Discussion and conclusions
In this study, we found that high Ki-67 LI was associated with a high APTw value. Ki-67 was widely used as a proliferation and prognostic factor of HCC. HCC with high Ki-67 LI tends to be more malignant with more mobile proteins. In addition, a higher Ki-67 index indicates a higher proliferation of hepatocellular carcinoma cells and greater cell density. Thus, the APTw value of HCC patients with high Ki-67 LI may also be high. Since APTw imaging can evaluate the expression of Ki-67 in HCC, APTw could be a non-invasive imaging method for preoperative diagnosis of HCC, which can provide an objective basis for the selection of treatment methods for clinical patients with HCC.In conclusion, this study confirmed that the APTw value and Ki-67 LI are significantly and positively correlated. APTw provides a potential method for non-invasive evaluation of the malignancy and proliferation of HCC cells. Follow-up studies with a larger patient cohort are needed to further validate the diagnostic performance of APTw.
Acknowledgements
We thank Weiqiang Dou from GE Healthcare for this valuable support on APT sequences.References
1 Lee JH, Yoon YC, Seo SW, Choi YL, Kim HS. Soft tissue sarcoma: DWI and DCE-MRI parameters correlate with Ki-67 labeling index. Eur Radiol. 2020;30(2):914-924.
2 Luo Y, Ren F, Liu Y, et al. Clinicopathological and prognostic significance of high Ki-67 labeling index in hepatocellular carcinoma patients: a meta-analysis. Int J Clin Exp Med. 2015;8(7):10235-10247.
3 Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085-1090.
4 Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50(6):1120-1126.
5 Li L, Chen W, Yan Z, et al. Comparative Analysis of Amide Proton Transfer MRI and Diffusion-Weighted Imaging in Assessing p53 and Ki-67 Expression of Rectal Adenocarcinoma. J Magn Reson Imaging. 2020;52(5):1487-1496.
6 Ochiai R, Mukuda N, Yunaga H, Kitao S, Okuda K, Sato S, Oishi T, Miyoshi M, Nozaki A, Fujii S. Amide proton transfer imaging in differentiation of type II and type I endometrial carcinoma: a pilot study. Jpn J Radiol. 2022 Feb;40(2):184-191.
7 Yu H, Wen X, Wu P, et al. Can amide proton transfer-weighted imaging differentiate tumor grade and predict Ki-67 proliferation status of meningioma?. Eur Radiol. 2019;29(10):5298-5306.
Figures