1808
Application of Deep Learning-based Reconstruction for Diffusion Kurtosis Imaging in Head and Neck Cancer
Amaresha Shridhar Konar1, Jaemin Shin2, Ramesh Paudyal1, Akash Deelip Shah3, Abhay Dave4, Maggie Fung2, Eve LoCastro1, Suchandrima Banerjee5, Nancy Lee6, and Amita Shukla-Dave1,3
1Medical Physics, Memorial Sloan Kettering Cancer Center, New York City, NY, United States, 2GE Healthcare, New York City, NY, United States, 3Radiology, Memorial Sloan Kettering Cancer Center, New York City, NY, United States, 4Touro College of Osteopathic Medicine, New York City, NY, United States, 5GE Healthcare, Menlo Park, CA, United States, 6Radiation Oncology, Memorial Sloan Kettering Cancer Center, New York City, NY, United States
1Medical Physics, Memorial Sloan Kettering Cancer Center, New York City, NY, United States, 2GE Healthcare, New York City, NY, United States, 3Radiology, Memorial Sloan Kettering Cancer Center, New York City, NY, United States, 4Touro College of Osteopathic Medicine, New York City, NY, United States, 5GE Healthcare, Menlo Park, CA, United States, 6Radiation Oncology, Memorial Sloan Kettering Cancer Center, New York City, NY, United States
Synopsis
Keywords: Quantitative Imaging, Machine Learning/Artificial Intelligence
A Deep learning (DL)-based reconstruction is a promising method to achieve higher resolution for diffusion-weighted Kurtosis imaging (DKI) without increasing signal averaging. The DKI phantom and patient results demonstrated improved image quality and reduced Gibbs (ringing) artifact, aiding in the robust estimation of Dapp and Kapp. In all phantom and patient data, the standard deviation of Dapp and Kapp measured in images reconstructed without DL was higher than in images reconstructed using DL. The NEX=1 significantly reduced the multi-b-value data acquisition time, and the DL-based reconstruction can produce images comparable to the standard NEX=2 or 4, depending on the b-value.Purpose:
Non-Gaussian diffusion kurtosis imaging (DKI) measures quantitative metrics describing the water diffusivity of hindered and restricted water molecules and tissue microstructure1,2. For extra-cranial regions such as the head and neck (HN), multi-b-value data acquisition is challenging, particularly at higher b-value with a reduced number of excitations, to maintain the optimal signal-to-noise ratio (SNR) required for DKI analysis2,3. A linear fit to the natural logarithm of the multiple b-value data with DKI modeling yields the apparent diffusion (Dapp) and kurtosis coefficient (Kapp), which are the surrogate markers of the tumor cellularity and tissue microstructure2. A recently developed novel deep learning (DL)-based MR reconstruction method (AIRTM Recon DL) has enhanced the image signal noise ratio (SNR), sharpness, and reduced truncation artifacts in prostate cancer patients4-6. Tumors in the HN region include a diverse group of cancers, and the accurate measurement of quantitative metrics may improve treatment response assessment for such tumors7-9. This study investigates whether DL-based reconstruction can improve the quantification of DKI metrics for tumors in the HN region.Methods:
MRI Data Acquisition: Data were acquired on a 3T MRI scanner (SIGNA Premier, GE Healthcare) using a 21-channel HN unit.Phantom: The novel, multi-exponential DKI phantom used in this study has two tiers of seven 20ml-glass scintillation vials containing lamellar vesicle (LV) solutions placed in a 1L jar filled with deionized water (Figure 2)10,11. The LV materials were made from fatty alcohols, surfactants, and water (solid-in-water %(w/w)=0.5%-2.5%). Two polyvinylpyrrolidone (PVP) at 20% and 40% were added as Gaussian-diffusion (Kapp=0) “controls.” The phantom contained an alcohol thermometer for temperature (T) reading to ±0.5°C. The phantoms were left to thermolyze in scanner rooms overnight before scanning, and the temperature during the exam was 19°C. The multi b-value DW images were acquired using a single shot spin echo planar imaging (SS-SE-EPI) sequence with TR/TE=4000/66 (minimum) ms, the field of view (FOV)=20 cm, matrix=128×128, slices=15, slice thickness=5mm, and b=0,20,50,80,200,300,500,1000,1500,2000 s/mm2. Two sets of data acquisition were performed using the above parameters with modification in the number of excitations (NEX): 1) Using the standard NEX=2 for b≤300 and NEX=4 for b≥500, and 2) Reducing the NEX=1 for all the b-values. The total scan time with the standard NEX was 6.12 min and for reduced NEX=1 was 2.13 min.
Patients: DW-MRI data were acquired from six HN cancer patients (median age 59 years, six males, 2 HPV(+) positive, 1 HPV(-), and 3 with unknown primary tumor status) in this retrospective study between December 2021 and June 2022. These patients underwent chemo-radiation therapy (CRT), and all MRI examinations were performed before treatment. MRI protocol consisted of multi-planar T1/T2 weighted imaging followed by multi-b-value DWI (b-values same as mentioned above) with TR/TE=4000/80 (minimum) ms, the field of view (FOV)=20-24 cm, matrix=128×128, slices=8-10, slice thickness=5 mm, number of excitation (NEX)=2 and b=0,20,50,80,200,300,500,1000,1500,2000 s/mm2. The raw data from the DW-MRI scans were transferred and retro-reconstructed using the AIRTM Recon DL algorithm in the GE reconstruction pipeline (Orchestra SDK, GE Healthcare) and finally labeled as DL images 4,5.
ROI Contouring and DWI Data Analysis: Regions of Interest (ROIs) were delineated on the primary tumors and neck nodal metastases by an experienced neuro-radiologist on both DW images with and without DL (b = 0 s/mm2) using ITK-SNAP. All DW data analysis was performed using in-house software, MRI-QAMPER (Quantitative Analysis Multi-Parametric Evaluation Routines), written in MATLAB (MathWorks, Natick, MA). ROI analysis yielded mean and standard deviation (STD) for each reported metric. Coefficient of variation (CV) and relative percent of difference reported.
Results:
The DL-based reconstructed images obtained from the DKI phantom showed reduced Gibbs (ringing) artifacts and increased image sharpness (Figure 1). Figure 2 exhibits the Dapp and Kapp maps for the standard and reduced NEX images reconstructed with and without DL for the DKI phantom. Dapp demonstrated no significant difference between the standard and reduced NEX images reconstructed using DL, while Kapp has shown a difference between the two acquisition and reconstruction strategies, particularly for vials #1, #6, and #7. The result of this comparison in the DKI phantom can be observed in Figure 3. Figure 4 shows data from a representative patient with primary tumor and neck nodal metastases. The improved SNR and reduced artifacts can be observed in the patient data, similar to phantom results (Figure 4). Table 1 summarizes the mean and CV of Dapp and Kapp in 10 ROIs for images with and without DL. For mean Dapp, a maximum difference of 5.22% was observed between the metrics derived from the images with and without DL, and for the mean Kapp, a maximum difference of 11.36% was observed between these two sets of images.Discussion and Conclusion:
The DL-based image reconstruction improved DKI image quality with increased sharpness and reduced Gibbs (ringing) artifacts. In all phantom and patient data, the STDs of Dapp and Kapp measured in images reconstructed without DL were higher than in images reconstructed using DL. Particularly at higher b-values, the DL-based image reconstruction improved image quality, reducing bias in estimated Dapp and Kapp. The NEX=1 significantly reduced the multi-b-value data acquisition time, and the DL-based reconstruction can produce images comparable to the standard NEX=2 or 4, depending on the b-value.Acknowledgements
Funding support from National Institutes of Health Grant: U01 CA211205 (ASD)References
- Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. Aug 2010;23(7):698-710. doi:10.1002/nbm.1518
- Jansen JF, Stambuk HE, Koutcher JA, Shukla-Dave A. Non-gaussian analysis of diffusion-weighted MR imaging in head and neck squamous cell carcinoma: A feasibility study. AJNR Am J Neuroradiol. Apr 2010;31(4):741-8. doi:10.3174/ajnr.A1919
- Solomon E, Lemberskiy G, Baete S, et al. Time-dependent diffusivity and kurtosis in phantoms and patients with head and neck cancer. Magn Reson Med. Oct 11 2022;doi:10.1002/mrm.29457
- Lebel RM. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. arXiv preprint arXiv:200806559. 2020;
- Choi M, Figee M, Lebel R, et al. Evaluation of the efficacy of a Deep Learning-based Reconstruction in the Connectomic Deep Brain Stimulation. Proc. Intl. Soc. Mag. Reson. Med. 30 (2022); 2022:3357.
- Ueda T, Ohno Y, Yamamoto K, et al. Deep Learning Reconstruction of Diffusion-weighted MRI Improves Image Quality for Prostatic Imaging. Radiology. 2022;303(2):373-381.
- Tshering Vogel DW, Thoeny HC. Cross-sectional imaging in cancers of the head and neck: how we review and report. Cancer Imaging. 2016;16(1):1-15.
- Paudyal R, Konar AS, Obuchowski NA, et al. Repeatability of Quantitative Diffusion-Weighted Imaging Metrics in Phantoms, Head-and-Neck and Thyroid Cancers: Preliminary Findings. Tomography. Mar 2019;5(1):15-25. doi:10.18383/j.tom.2018.00044
- Riaz N, Sherman E, Pei X, et al. Precision Radiotherapy: Reduction in Radiation for Oropharyngeal Cancer in the 30 ROC Trial. JNCI: Journal of the National Cancer Institute. 2021;113(6):742-751. doi:10.1093/jnci/djaa184
10. D. Malyarenko TC, S. Ono, T. Lynch, S. Swanson. Temperature and Concentration Dependence of Diffusion Kurtosis Parameters in a Quantitative Phantom. 2022:2429.
11. Solomon E, Lemberskiy G, Baete S, et al. Time‐dependent diffusivity and kurtosis in phantoms and patients with head and neck cancer. Magnetic Resonance in Medicine. 2022;
Figures
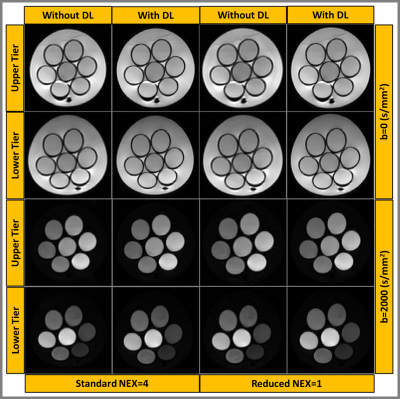
Figure 1:
Representative images (b=0 and 2000 s/mm2) from the upper and lower tier
of Diffusion Kurtosis Imaging (DKI) phantom reconstructed with and without DL.
Two series of acquisitions were performed using standard (NEX=2 and 4) and reduced
(NEX=1) number of excitations for multi b-value DKI for comparing the image
quality.
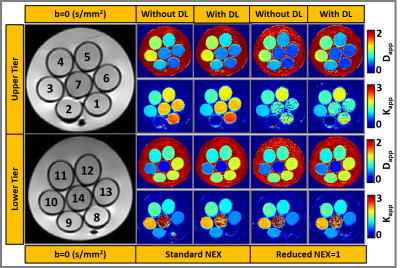
Figure 2: Selected
slices from the upper and lower tier of DKI phantom with 7 vials in each layer.
Multi-b-value DW-MRI data reconstructed with and without DL for estimating Dapp
(x10-3
mm2/s) and Kapp.
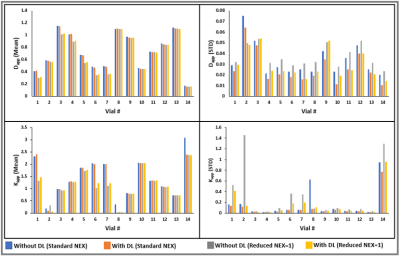
Figure 3: Mean and
standard deviation (STD) of Dapp and Kapp measured from
14 vials in the DKI phantom.
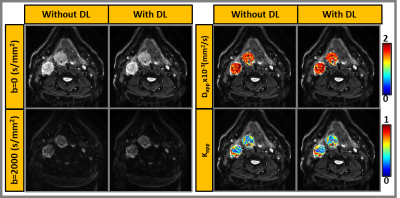
Figure 4: Representative
patient showing primary tumor and neck nodal metastases. Images reconstructed with
and without DL for selected b-values (b=0 and b=2000 s/mm2). Dapp and Kapp
maps overlaid on b=0 images.
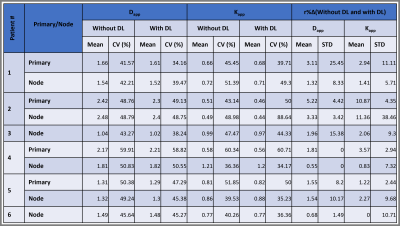
Table 1: Summary of the mean and coefficient of variation (CV in %) of measured Dapp and Kapp from images reconstructed
with and without DL for patient data.
DOI: https://doi.org/10.58530/2023/1808