1799
Application of six diffusion-weighted MRI models in predicting lymph node metastasis for resectable gastric cancer: A pilot study
Jing Li1, Shao-yu Wang2, and Xue-jun Chen1
1Radiology, the Affiliated Cancer Hospital of Zhengzhou University (Henan Cancer Hospital), Zhengzhou, China, 2MR Scientific Marketing, Siemens Healthneers, Shanghai, Shanghai, China
1Radiology, the Affiliated Cancer Hospital of Zhengzhou University (Henan Cancer Hospital), Zhengzhou, China, 2MR Scientific Marketing, Siemens Healthneers, Shanghai, Shanghai, China
Synopsis
Keywords: Quantitative Imaging, Tumor
This study firstly explored the potential of six diffusion-weighted MRI models for preoperative prediction of lymph node metastasis (LNM) in resectable gastric cancer (GC). The DKI_K, CTRW_D, DKI_D, FROC_D, IVIM_D*, IVIM_f, Mono_ADC, as well as morphologic indicators of tumor thickness, clinical T staging on MRI (cT), MRI reported LN status were significantly different between LNM positive and LNM negative groups. These parameters were statistically correlated with LNM. The cT, MRI reported LN, and CTRW_D were independent risk factors of LNM, combining these three parameters yielded the highest predictive efficacy.Purpose
The aim of this study was to study and compare the potentials of different parameters derived from six diffusion-weighted MRI models in predicting lymph node metastasis (LNM) for resectable gastric cancer (GC).Methods
This study prospectively included 58 consecutive GC patients who received standard radical D2 gastrectomy plus lymph nodes dissection (41 males, 17 females) in the age group of 39–77 years (average, 60.88±10.48 years). All the patients underwent un-contrasted and dynamic contrasted enhanced MRI scans on stomach, including multi b value DWI sequence, within 1 week before surgery. Taking pathologic diagnosis of LNM as the ground truth, patients were divided into LNM positive and LNM negative groups. Six sequences were derived from multi b value DWI, including continuous time random walk diffusion-weighted imaging (CTRW), Diffusion kurtosis imaging (DKI), fractional order calculus diffusion (FROC), intravoxel incoherent motion diffusion-weighted imaging (IVIM), conventional mono-exponential DWI, stretched exponential model (SEM). The differences of relevant parameters including CTRW_α, CTRW_β, DKI_D, DKI_K, FROC_D, FORC_β, FROC_mμ, IVIM_D, IVIM_D*, IVIM_f, SEM_α, IVIM_DDC, were compared between the two groups. Morphological MRI features and clinicopathological characteristics were also analyzed. Multivariable logistic regression was used to identify independent predictors for LNM, whereas receiver-operating characteristic curve analyses were applied to evaluate the efficacy, the sensitivities, specitifitie, positive predictive value (PPV), negative predictive value (NPV), the cut off value were calculated. The correlations between significant parameters and LNM were explored through Spearman and Pearson correlation tests.Results
The tumor thickness and DKI_K in the LNM positive group were higher than those in the LNM negative group, whereas CTRW_D, DKI_D, FROC_D, IVIM_D*, IVIM_f, Mono_ADC in LNM positive group were lower than those in LNM negative group (P<0.05). The MRI reported LN and cT were statistically different between the two groups (P<0.05). These above parameters showed statistical correlations with LNM. The cT, MRI reported LN, and CTRW_D were independent influencing parameters of LNM. The combined parameter (cT+MRI reported LN+CTRW_D) yielded significantly higher efficacy than any other individual parameters for preoperative prediction of LNM in GC (Delong test, all P < 0.05).Conclusions
The CTRW_D, DKI_D, FROC_D, IVIM_D*, IVIM_f, Mono_ADC are correlated with LNM, and can effectively distinguish LNM status in GC. The cT, MRI reported LN, and CTRW_D are independent predictors of LNM. The combination of the three parameters further improve the predictive efficacy.Acknowledgements
No acknowledgement found.References
- Sui Y, Wang H, Liu G, Damen FW, Wanamaker C, Li Y, Zhou XJ. Differentiation of Low- and High-Grade Pediatric Brain Tumors with High b-Value Diffusion-weighted MR Imaging and a Fractional Order Calculus Model. Radiology. 2015 Nov;277(2):489-96. doi: 10.1148/radiol.2015142156.
- Gatto RG, Ye AQ, Colon-Perez L, Mareci TH, Lysakowski A, Price SD, Brady ST, Karaman M, Morfini G, Magin RL. Detection of axonal degeneration in a mouse model of Huntington's disease: comparison between diffusion tensor imaging and anomalous diffusion metrics. MAGMA. 2019 Aug;32(4):461-471. doi: 10.1007/s10334-019-00742-6
- Shao X, An L, Liu H, Feng H, Zheng L, Dai Y, Yu B, Zhang J. Cervical Carcinoma: Evaluation Using Diffusion MRI With a Fractional Order Calculus Model and its Correlation With Histopathologic Findings. Front Oncol. 2022 Apr 5;12:851677. doi: 10.3389/fonc.2022.851677.
- Karaman MM, Tang L, Li Z, Sun Y, Li JZ, Zhou XJ. In vivo assessment of Lauren classification for gastric adenocarcinoma using diffusion MRI with a fractional order calculus model. Eur Radiol. 2021 Aug;31(8):5659-5668. doi: 10.1007/s00330-021-07694-3.
- Chen J, Guo Y, Guo Y, Jiang M, Zhang Y, Dai Y, Yao X. Preoperative assessment of microvascular invasion of hepatocellular carcinoma using non-Gaussian diffusion-weighted imaging with a fractional order calculus model: A pilot study. Magn Reson Imaging. 2021 Sep 8:S0730-725X(21)00144-2. doi: 10.1016/j.mri.2021.09.003.
- Li Z, Dan G, Tammana V, Johnson S, Zhong Z, Rabiee B, Zhou XJ, L Xie K. Predicting the aggressiveness of peripheral zone prostate cancer using a fractional order calculus diffusion model. Eur J Radiol. 2021 Oct;143:109913. doi: 10.1016/j.ejrad.2021.109913.
- Feng C, Wang Y, Dan G, Zhong Z, Karaman MM, Li Z, Hu D, Zhou XJ. Evaluation of a fractional-order calculus diffusion model and bi-parametric VI-RADS for staging and grading bladder urothelial carcinoma. Eur Radiol. 2022 Feb;32(2):890-900. doi: 10.1007/s00330-021-08203-2.
- Qin Y, Tang C, Hu Q, Yi J, Yin T, Ai T. Assessment of Prognostic Factors and Molecular Subtypes of Breast Cancer With a Continuous-Time Random-Walk MR Diffusion Model: Using Whole Tumor Histogram Analysis. J Magn Reson Imaging. 2022 Oct 17. doi: 10.1002/jmri.28474.
- Karaman MM, Zhang J, Xie KL, Zhu W, Zhou XJ. Quartile histogram assessment of glioma malignancy using high b-value diffusion MRI with a continuous-time random-walk model. NMR Biomed. 2021 Apr;34(4):e4485. doi: 10.1002/nbm.4485.
- Fu J, Tang L, Li ZY, Li XT, Zhu HF, Sun YS, Ji JF. Diffusion kurtosis imaging in the prediction of poor responses of locally advanced gastric cancer to neoadjuvant chemotherapy. Eur J Radiol. 2020 Jul;128:108974. doi: 10.1016/j.ejrad.2020.108974.
- Shi B, Yuan F, Yan F, Zhang H, Pan Z, Chen W, Wang G, Tan J, Zhang Y, Ren Y, Du L. Evaluation of Effects of TGF-β1 Inhibition on Gastric Cancer in Nude Mice by Diffusion Kurtosis Imaging and In-Line X-ray Phase Contrast Imaging With Sequential Histology. J Magn Reson Imaging. 2019 Jun;49(6):1553-1564. doi: 10.1002/jmri.26523.
- Zhou Z, Chen Y, Zhao F, Sun Z, Zhu L, Yu H, Wang W. Predictive value of intravoxel incoherent motion combined with diffusion kurtosis imaging for breast cancer axillary lymph node metastasis: a retrospective study. Acta Radiol. 2022 Jun 28:2841851221107626. doi: 10.1177/02841851221107626.
- Yamada I, Sakamoto J, Kobayashi D, Miyasaka N, Wakana K, Oshima N, Wakabayashi A, Saida Y, Tateishi U, Eishi Y. Diffusion kurtosis imaging of endometrial carcinoma: Correlation with histopathological findings. Magn Reson Imaging. 2019 Apr;57:337-346. doi: 10.1016/j.mri.2018.12.009.
- Liu S, Guan W, Wang H, Pan L, Zhou Z, Yu H, Liu T, Yang X, He J, Zhou Z. Apparent diffusion coefficient value of gastric cancer by diffusion-weighted imaging: correlations with the histological differentiation and Lauren classification. Eur J Radiol. 2014 Dec;83(12):2122-2128. doi: 10.1016/j.ejrad.2014.09.021.
- Zeng Q, Hong Y, Cheng J, Cai W, Zhuo H, Hou J, Wang L, Lu Y, Cai J. Quantitative study of preoperative staging of gastric cancer using intravoxel incoherent motion diffusion-weighted imaging as a potential clinical index. Eur J Radiol. 2021 Aug;141:109627. doi: 10.1016/j.ejrad.2021.109627.
- Seo N, Chung YE, Park YN, Kim E, Hwang J, Kim MJ. Liver fibrosis: stretched exponential model outperforms mono-exponential and bi-exponential models of diffusion-weighted MRI. Eur Radiol. 2018 Jul;28(7):2812-2822. doi: 10.1007/s00330-017-5292-z.
- Chen X, Jiang J, Shen N, Zhao L, Zhang J, Qin Y, Zhang S, Li L, Zhu W. Stretched-exponential model diffusion-weighted imaging as a potential imaging marker in preoperative grading and assessment of proliferative activity of gliomas. Am J Transl Res. 2018 Aug 15;10(8):2659-2668.
- Park JH, Seo N, Chung YE, Kim SU, Park YN, Choi JY, Park MS, Kim MJ. Noninvasive evaluation of liver fibrosis: comparison of the stretched exponential diffusion-weighted model to other diffusion-weighted MRI models and transient elastography. Eur Radiol. 2021 Jul;31(7):4813-4823. doi: 10.1007/s00330-020-07600-3.
- Lin M, Yu X, Chen Y, Ouyang H, Wu B, Zheng D, Zhou C. Contribution of mono-exponential, bi-exponential and stretched exponential model-based diffusion-weighted MR imaging in the diagnosis and differentiation of uterine cervical carcinoma. Eur Radiol. 2017 Jun;27(6):2400-2410. doi: 10.1007/s00330-016-4596-8.
- Liu C, Wang K, Li X, Zhang J, Ding J, Spuhler K, Duong T, Liang C, Huang C. Breast lesion characterization using whole-lesion histogram analysis with stretched-exponential diffusion model. J Magn Reson Imaging. 2018 Jun;47(6):1701-1710. doi: 10.1002/jmri.25904.
- Lai V, Lee VH, Lam KO, Sze HC, Chan Q, Khong PL. Intravoxel water diffusion heterogeneity MR imaging of nasopharyngeal carcinoma using stretched exponential diffusion model. Eur Radiol. 2015 Jun;25(6):1708-13. doi: 10.1007/s00330-014-3535-9.
- Suo S, Yin Y, Geng X, Zhang D, Hua J, Cheng F, Chen J, Zhuang Z, Cao M, Xu J. Diffusion-weighted MRI for predicting pathologic response to neoadjuvant chemotherapy in breast cancer: evaluation with mono-, bi-, and stretched-exponential models. J Transl Med. 2021 Jun 2;19(1):236. doi: 10.1186/s12967-021-02886-3.
- Anderson SW, Barry B, Soto J, Ozonoff A, O'Brien M, Jara H. Characterizing non-gaussian, high b-value diffusion in liver fibrosis: Stretched exponential and diffusional kurtosis modeling. J Magn Reson Imaging. 2014 Apr;39(4):827-34. doi: 10.1002/jmri.24234.
Figures
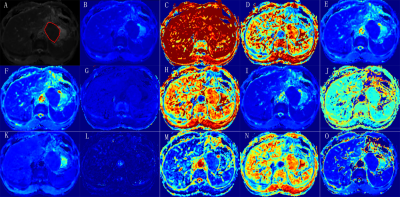
Parameters maps derived from six diffusion-weighted MRI models in a
LNM positive patients with pathologically confirmed gastric adenocarcinoma by
radical gastrectomy, pT3N1M0. (A) Mono_ADC grayscale map with tumor
segmentation; (B) Mono_ADC jet map; (C) CTRW_α map; (D) CTRW_β map; (E) CTRW_D
map; (F) DKI_D map; (G) DKI_K map; (H) FROC_β map; (I) FROC_D map; (J) FROC_ mμ
map; (K) IVIM_D map; (L) IVIM_D* map; (M) IVIM_f map; (N) SEM_α map; (O) SEM_DDC map.
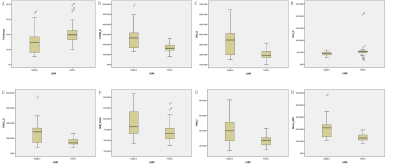
Box
plots diagramS of significant MRI parameters between LNM negative group and LNM
positive group.
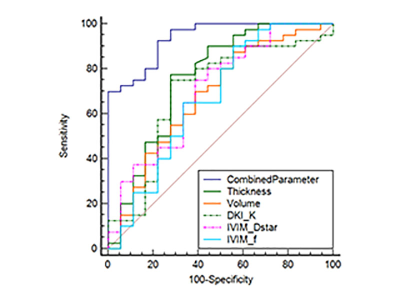
Comparison among sole significant
parameters and the combined parameter (cT+ MRI reported LN+CTRW_D).
DOI: https://doi.org/10.58530/2023/1799