1798
Diffusion Tensor Imaging Parameters in ALS Post Mortem In Situ versus Ex Situ MR Acquisitions1Institute of Forensic Medicine, Dept. of Biomedical Engineering, University of Basel, Basel, Switzerland, 2Institute of Forensic Medicine, Health Department Basel-Stadt, Basel, Switzerland, 3Neurology Clinic and Polyclinic, Dept. of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland, 4Translational Imaging in Neurology (ThINk), Dept. of Biomedical Engineering, University of Basel, Basel, Switzerland, 5Division of Radiological Physics, Dept. of Radiology and Nuclear Medicine, University Hospital Basel, Basel, Switzerland
Synopsis
Keywords: Quantitative Imaging, Diffusion Tensor Imaging
The development of magnetic resonance imaging (MRI) biomarkers in neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) might alleviate current challenges. Generally, post mortem (PM) ex situ quantitative imaging is used for the validation of potential biomarkers, which shows several limitations. In this study, PM MRI brain scans of three deceased ALS patients have been conducted. The objective was to identify differences between in situ and ex situ scans regarding the diffusion tensor imaging (DTI) measures fractional anisotropy (FA) and mean diffusivity (MD). A significant difference was found for the FA values in white matter.INTRODUCTION
Magnetic resonance imaging (MRI) biomarkers may support the diagnosis and help estimate the prognosis of neurodegenerative diseases like amyotrophic lateral sclerosis (ALS)1. Potential biomarkers need to be validated to characterise tissue properties and identify disease-related tissue alterations and their respective imaging signature2.However, the majority of studies incorporating a validation directly correlate the in vivo MRI findings with post mortem (PM) ex situ MRI measures acquired in formalin fixed tissue, which has several limitations like change of volume or altered quantitative MRI values3-5. For a proper validation, it is therefore crucial to understand tissue changes occurring from extraction and formalin fixation only. These have to be differentiated from disease-related changes.
The goal of this study was to evaluate the differences between PM in situ and PM ex situ cerebral MRI measurements focusing on fractional anisotropy (FA) and mean diffusivity (MD) in patients with ALS.
METHODS
To date, three deceased patients with a clinical diagnosis of definite ALS (according to the revised El Escorial criteria6) have been included in this study. The patients agreed to participate in this study during lifetime (ethical approval: BASEC-Nr. 2020-02179). Post mortem (PM) in situ as well as ex situ MRI brain scans have been performed on all patients.In situ: Intact body. Scan performed within 24h after time of death. Prior to the scan, the body was placed in a cooling chamber at 4°C and the brain temperature was measured via a probe through the os ethmoidale.
Ex situ: The brains were extracted the day after the in situ scan and immersed in a 4.5% formalin solution for three months. For the scan, the brains were mounted in patient-specific 3D printed holders and placed in a spherical container. The container was then filled with a proton-free and tissue susceptibility matched fluid (Galden®, Solvay SA).
The scans were performed on a 3 T Siemens MAGNETOM Prisma device and included anatomical MP2RAGE7 as well as diffusion tensor imaging (DTI) scans.
MP2RAGE: 176 slices per slab, FoV = 240 × 256 mm, TR = 5000 ms, TE = 2.98 ms, TI = 700 and 2500 ms, flip angle = 4° and 5°, GRAPPA acceleration factor 3 in 08:20min, isotropic resolution of (1x1x1) mm3.
DTI: b = 2000 s/mm2, 64 isotropically distributed diffusion directions, 3 b = 0 s/mm2, TE = 109 ms, TR = 18.700 ms, 100 slices, isotropic resolution of (1.8x1.8x1.8) mm3.
The data were processed using 3D Slicer8 for masking and FSL (the FMRIB Software Library)9 for segmentation, registration, as well as FA and MD analyses. FA and MD values were compared between in situ and ex situ conditions in the whole brain grey and white matter, respectively. The MD in situ values were corrected for temperature to match the ex situ conditions as described by Berger et al.10.
For each diffusion measure, all in situ mean values were compared with all ex situ mean values, using an independent two-sample t-test (two-tailed, variance evaluated for each case). The significance level alpha was defined as 5%.
RESULTS
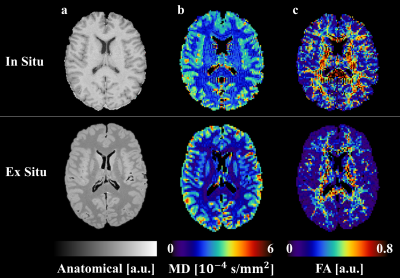
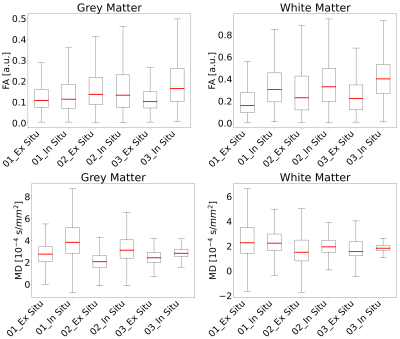
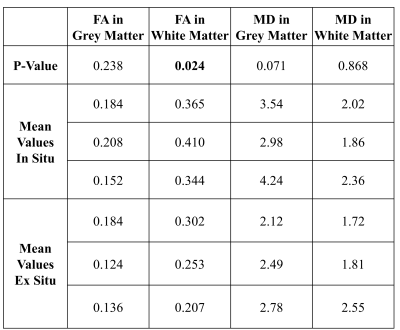
For illustration, the parameter maps of one ALS patient are shown in Figure 1. The distribution of the MD and FA values of all participants measured in- and ex situ in brain grey - and white matter are shown in Figure 2. FA measured in white matter in situ was significantly higher than the corresponding FA measured ex situ (p=0.024). No significant differences were found for FA in grey matter and MD in white and grey matter. The corresponding p-values and mean values are depicted in Table 1.DISCUSSION
In this study, we compared FA and MD in brain grey and white matter between PM in situ and PM ex situ MRI scans in deceased patients with a clinical diagnosis of definite ALS. Significant differences between the in- and ex situ PM conditions were found for the FA values in white matter. Mean diffusivity in grey matter was slightly higher in in situ compared to ex situ scans, though not statistically significant (p=0.07), and should therefore also be carefully evaluated when running PM ex situ scans of formalin fixed brains.We also noticed changes in brain shape between in situ and ex situ scans.
The results presented here are based on only three ALS cases scanned both in situ within 24 hours after death and ex situ. Both the small sample size as well as the lack of a healthy control group are limitations of the study.
CONCLUSION
The results of this study suggest that FA values in brain white matter and potentially also MD values in brain grey matter in ALS brains are altered by the extraction and fixation process compared to the in situ scan obtained directly after death. FA values in grey matter, as well as the MD values in white matter retrieved in PM ex situ MRI scans were not significantly different from the in situ data sets. Studying a larger sample will be necessary for confirmation of our results and to, e.g., derive a correction model for the observed alterations.Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.References
1 Barritt, A. W., Gabel, M. C., Cercignani, M. & Leigh, P. N. Emerging Magnetic Resonance Imaging Techniques and Analysis Methods in Amyotrophic Lateral Sclerosis. Front Neurol 9, 1065, doi:10.3389/fneur.2018.01065 (2018).
2 Alyami, W., Kyme, A. & Bourne, R. Histological Validation of MRI: A Review of Challenges in Registration of Imaging and Whole-Mount Histopathology. J Magn Reson Imaging 55, 11-22, doi:10.1002/jmri.27409 (2022).
3 Maranzano, J. et al. A novel ex vivo, in situ method to study the human brain through MRI and histology. J Neurosci Methods 345, 108903, doi:10.1016/j.jneumeth.2020.108903 (2020).
4 Schulz, G. et al. Three-dimensional strain fields in human brain resulting from formalin fixation. J Neurosci Methods 202, 17-27, doi:10.1016/j.jneumeth.2011.08.031 (2011).
5 Shatil, A. S., Uddin, M. N., Matsuda, K. M. & Figley, C. R. Quantitative Ex Vivo MRI Changes due to Progressive Formalin Fixation in Whole Human Brain Specimens: Longitudinal Characterization of Diffusion, Relaxometry, and Myelin Water Fraction Measurements at 3T. Front Med (Lausanne) 5, 31, doi:10.3389/fmed.2018.00031 (2018).
6 Brooks, B. R., Miller, R. G., Swash, M., Munsat, T. L. & World Federation of Neurology Research Group on Motor Neuron, D. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1, 293-299, doi:10.1080/146608200300079536 (2000).
7 Marques, J. P. et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49, 1271-1281, doi:10.1016/j.neuroimage.2009.10.002 (2010).
8 Kikinis, R., Pieper, S. D. & Vosburgh, K. G. in Intraoperative Imaging and Image-Guided Therapy Ch. Chapter 19, 277-289 (2014).
9 Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. Fsl. Neuroimage 62, 782-790, doi:10.1016/j.neuroimage.2011.09.015 (2012).
10 Berger, C., Bauer, M., Wittig, H., Scheurer, E. & Lenz, C. Post mortem brain temperature and its influence on quantitative MRI of the brain. MAGMA, doi:10.1007/s10334-021-00971-8 (2021).Figures