1796
Accelerated Sampling Strategy for High-resolution 3D T1rho Dispersion MRI with 4D Dynamic Radial Acquisitions1Department of Radiology, Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, NY, United States, 2Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Philips Healthcare, Cambridge, MA, United States
Synopsis
Keywords: Quantitative Imaging, Quantitative Imaging, T1rho dispersion imaging
T1ρ dispersion imaging based on repeated T1ρ measurements at multiple spin-lock frequencies allows characterization of different dynamic processes of tissues. The clinical potential of these techniques is, however, limited by the total scan time needed to obtain reliable and consistent quantitative measurements. In this abstract, we propose the application of a 4D dynamic sequence with radial acquisitions in combination with an accelerated data-point sampling strategy to achieve efficient coverage of the high-dimensional data space in T1rho dispersion MRI, which leads to high-resolution 3D T1rho dispersion imaging acquired within clinically acceptable scan duration of ~5 minutes.Introduction
The spin-lattice relaxation time in the rotating frame (T1ρ, and the relaxation rate R1ρ = 1/T1ρ) measured from a few spin-lock time (TSL) at a single spin-lock frequency (FSL, e.g., 500Hz), has been shown to be sensitive to tissue abnormalities. How T1ρ varies as a function of FSL (T1ρ dispersion) additionally illustrates the functional and dynamic properties of tissue [1-3]. However, T1ρ dispersion imaging sequences demand long acquisition time, since multiple data-point acquisitions to cover both TSL-FSL directions are needed. A few fast-imaging approaches have been proposed recently for high-resolution 3D T1ρ dispersion imaging, dramatically reducing scan time [4-6]. However, there has been no study on optimization of data-point sampling in the FSL-TSL space for accelerated imaging. In this work, we propose the application of 4D radial acquisitions with accelerated sampling strategy for high-spatial resolution 3D T1ρ dispersion MRI, leading to feasible scan duration in the clinical setting.Accelerated Sampling Strategy
4D contrast-enhanced dynamic imaging sequences are among the most important sequences in the clinical environment to obtain high temporospatial resolution imaging to best delineate the contrast-enhancing dynamics in tissues post-injection. These sequences are typically vastly under-sampled for fast imaging, optimized based on the expected signal evolution along the time dimension. To allow faster imaging with minimal signal distortion during T1ρ dispersion imaging, we propose the data-point sampling strategy as shown in Figure. 1. The sampling order of data-points (red arrows) in the TSL-FSL sampling space has been optimized to minimize signal change along the data acquisition train. We applied the strategy in a vender-specific dynamic 4D radial acquisition sequence (Philips 4D Freebreathing, termed “4DVane” herein), leading to an accelerated sequence termed “Fast 4DVane” herein.Imaging and Processing Methods
The basic T1ρ dispersion imaging sequence is based on an unpaired PC MAPSS sequence in combination with T1ρ preparations at different TSLs and FSLs, as presented previously [4,6]. Three pulse sequences were compared in this work. The first applies traditional Cartesian readout in combination with compressed sensing reconstruction, termed “Cartesian” method herein. The second method uses 4DVane sequence with full data sampling along the FSL-TSL directions. The third sequence is Fast 4DVane with view-sharing along the FSL direction as shown in Figure. 1. All imaging experiments were performed on a 3T Philips Ingenia MR scanner with a 1ch-TX/16ch-Rx knee coil. Axial 3D volumetric acquisitions had the following scan parameters: FOV=140/140/180mm3, acquisition voxel size=1×1×4mm3, TR/TE=5.4/2.6ms, recovery delay time=1s, and GRE readout train length=96 with centric profile ordering [5]. Compressed SENSE factor was 3 for the “Cartesian” method, and SENSE factor was 1.3 along Kz-direction for the two 4DVane sequences. The same sequences were tested both on a plant phantom (sweet potato+orange) and in vivo on calf muscles of a healthy volunteer. All sequences were performed with 10 FSLs from 0 to 900Hz (100Hz gap) and 3 TSLs (0+, 25+, 35- ms), where ± represents positive/negative phase cycling. The total scan durations for the Cartesian, 4DVane, and fast 4DVane sequences were 9:00, 9:25, and 5:22 min, respectively. T1ρ maps at different FSLs were iteratively reconstructed voxel-by-voxel using a mono-exponential signal model using complex data as described in [6].Results
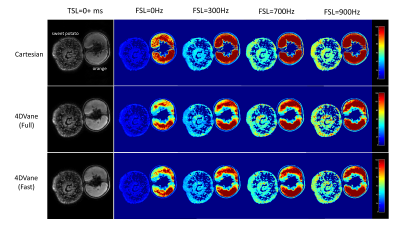
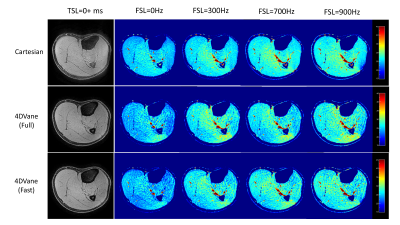
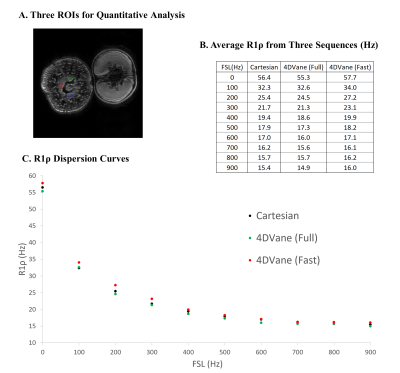
All three methods (Cartesian, 4DVane, and Fast 4DVane) generated high-spatial resolution T1ρ dispersion imaging in both the plant phantom and human studies. Figure 2 shows the comparison between T1ρ maps at different FSLs of the plant phantom. There is little difference between the T1ρ maps from the sweet potato. T1ρ values have the same trend in all sequences but larger differences for the orange due to its much larger T1ρ values than the designed range of measurement. Figure 3 shows the results from calf muscles at different FSLs. There is little difference between the T1ρ maps, and all sequences had the same upward trend at higher FSL. Figure 4 shows the quantitative results of R1ρ and the R1ρ dispersion curves of the average R1ρ from ROIs on the sweet potato. The R1ρ dispersion curves are highly consistent among all methods, despite the R1ρ values varied much larger than typical in physiological tissues. Human muscle results (not shown) also had similar consistency with much less R1ρ variability at different FSLs.Discussions
T1ρ dispersion imaging based on T1ρ measurements at multiple FSLs allows characterization of different dynamic processes of tissues. The clinical potential of T1ρ dispersion imaging is, however, limited by the long scan time needed for reliable and consistent measurements with 3D volume coverage. To reduce total imaging time, we propose the application of a dynamic 4D sequence with radial acquisitions for accelerated T1ρ dispersion imaging. We additionally applied an optimized data-point sampling strategy to minimize expected signal change along the data acquisition train, which allows more aggressive view sharing. We demonstrated the feasibility of this approach using the state-of-the-art fast MAPSS sequence [4] on both the plant phantom and human studies to achieve high-quality 3D T1ρ dispersion imaging at ~5min with 10 FSLs and three TSLs. Further scan time reduction may be achieved by applying compressed sensing reconstruction in the Fast 4DVane sequence. Model- or AI-based reconstruction methods, which generate the T1ρ dispersion curve directly, potentially only need a fraction of the data acquired here.Acknowledgements
No acknowledgement found.References
1. Cobb JG, Xie J, Gore JC. Contributions of chemical and diffusive exchange to T1rho dispersion. Magn Reson Med. 2013;69(5):1357-1366.
2. Koskinen SK, Virta AM, Niemi PT, et al. T1rho dispersion of rat tissues in vitro. Magn Reson Imaging. 1999;17(7):1043-1047.
3. Wang P, Block J, Gore JC. Chemical exchange in knee cartilage assessed by R1rho (1/T1rho) dispersion at 3T. Magn Reson Imaging. 2015;33(1):38-42.
4. Peng Q, Wu C, Kim J, Li X. Efficient phase-cycling strategy for high-resolution 3D gradient-echo quantitative parameter mapping. NMR Biomed 2022; 35(7): e4700.
5. Peng Q, Wu C. Fast 3D T1rho Dispersion MRI with Interleaved Phase Cycling MAPSS, Proc Intl Soc Mag Reson Med 30, London, 2022.
6. Peng Q, Peng GW, Wu C. Integrated T1rho Dispersion Imaging and Quantification, Proc Intl Soc Mag Reson Med 30, London, 2022.
7. Peng GW, Wu C, Peng Q. Improved Phase-cycling Preparations in Quantitative T1rho Mapping, Proc Intl Soc Mag Reson Med 30, London, 2022.
Figures