1787
Low Volume, Pneumatic Flow Phantom for Validation of Flowing T2* Estimation Using Projection Acquisition with Flow-Encoded Subtraction1Department of Biomedical Engineering, University of Texas at Austin, Austin, TX, United States, 2Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 3Department of Radiology, Stanford University, Stanford, CA, United States
Synopsis
Keywords: Quantitative Imaging, Phantoms, non-cartesian
Blood-oxygen level is a known biomarker for cardiovascular diseases which can be quantified by T2*-weighted MRI. Challenges with validation and motion have limited the clinical use of T2* oximetry methods. We present a PALMS sequence to measure flowing T2* which was validated using a pneumatically powered, low-volume, continuous flow phantom. Our results suggest that the presence of readout gradients influence T2* during flowing conditions while obliquity influences T2* in all settings conditions.Introduction
MR-oximetry encompasses a class of techniques that can be used to non-invasively determine the intravascular venous oxygen saturation (SVO2) of large conduit vessels by leveraging the intrinsic susceptibility difference between oxy- and deoxyhemoglobin1.MR-oximetry has been used to quantify brain svo2, oxygen extraction fraction and even cerebral metabolic rate (CMRO2) when combined with blood flow imaging. Unfortunately, MR-oximetry techniques are difficult to validate and have failed to see clinical use. Many techniques require calibration equations or models that are setting, subject, and sequence dependent, poorly characterized in vivo and confounded by pathology2. The clinical standard validation involves invasive blood gas sampling of deep venous structure (i.e., the sagittal sinus) which is beyond minimal risk and is difficult to perform. Ex vivo validations in blood require complex experimental setups controlling for temperature, oxygenation, and sedimentation, whereas validation with contrast agent doped fluids are rarely performed in the presence of flow. Furthermore, speed and motion sensitivity have historically limited the use of MR-oximetry to brain applications despite utility in many organs of the body i.e., heart, lungs and renal vessels.
In order to address these limitations, we developed a low-fluid volume, pneumatic flow phantom and a motion robust, non-cartesian T2* based sequence for flowing, intravascular T2* quantification.
Methods
Pneumatic Flow PhantomTypical flow phantoms utilize electric pumps with ferrous components that must be placed in the MR control room and large volumes of fluid are pumped into the scanner suite. Ex vivo validation often requires many gallons of blood or fluid at various oxygen tension and/or contrast agent concentrations. To overcome this challenge, we constructed a flow phantom that operates on less than 1.5 liters of fluid. An illustrative and schematic depiction of the phantom is found in Figure 1. A pneumatic turbine was attached via a drive shaft to a submerged impeller housed in a .5 L reservoir. An air compressor outside the magnet room drove the turbine, creating a centrifugal force and positive pressure inside the impeller housing that pumped water into a second reservoir. The second reservoir was attached via agar lined vinyl tubing downstream to the reservoir containing the impeller system. When the air compressor was on, a water height pressure differential was created by the impeller system that enabled continuous fluid flow. Continuous flow was measured outside the magnet room using an electronic, inline flowmeter.
PALMS Sequence
We built a 2D projection acquisition with flow-encoded, multi-echo subtraction (PALMS) sequence to generate intravascular T2* estimates. The PALMS sequence was modeled after prior MR-oximetry techniques3, and combined phase contrast encoding and subtraction for flowing fluid isolation, a radial acquisition for motion robustness and a free-induction decay (FID) multi-echo sampling scheme for T2* estimates uncorrupted by flow. The TR was 48 milliseconds (ms). Four FID echoes were acquired with a golden-angle, full spoke radial projection at echo times of 3.7, 17.7, 31.7 and 45.7 ms. Each FID was sampled with 400 radial spokes. Each spoke was acquired with tetrahedral flow encoding for velocity estimation and flow isolation in all principal directions. The total scan time was 5 minutes and 20 seconds. Radial spokes of velocity and FID echoes were binned and a parallel imaging reconstruction with locally low-rank regularization was performed in BART4.
Distilled water, doped with ferumoxytol at multiple concentrations was used to determine T2* estimates as compared to a standard, Cartesian multi-echo sequence during static and flowing conditions. Imaging was performed in a healthy volunteer as a proof of concept evaluation.
Results
The pneumatic phantom generated a constant flow rate, stable over multiple hours (Figure 3). In the presence of flow, a standard, multi-echo Cartesian sequence yielded inaccurate T2* values (slope = 8.5, R2 = 0.85) as compared non-flowing conditions. In comparison, T2* values measured using PALMS during flowing conditions, had a strong correlation to those measured under static conditions with a standard Cartesian sequence (slope = 1.03 R2 = 0.99). Vessel obliquity with respect to the main B0 field caused T2* values to diverge as compared to B0 parallel cases. However, the divergent T2* values were the same in oblique, flowing PALMS estimates and oblique static Cartesian measurements (Slope = 0.81, R2 = 0.99). PALMS in the abdomen of a healthy subject showed good flow isolation and motion robustness during free breathing.Discussion
We demonstrate that a pneumatic flow phantom generates stable continuous fluid flow and is useful for intravascular T2* validation. We also show a novel PALMS sequence can be used to make accurate T2* estimates in flowing conditions. Unfortunately, the T2* estimates were influenced by the obliquity of the vessel which may limit the ability for T2* to be used in quantitative MR-oximetry. The obliquity dependence is likely due to the well described orientation dependent susceptibility fields created by cylindrical tubes. PALMS T2* estimates may be useful in future applications where motion robust, qualitative intravascular oximetry is required.Acknowledgements
This work was supported by 5R01EB026136-03, 5R01EB009690-11 and start-up funds at The University of Texas at Austin.References
1. Wright GA, Hu BS, Macovski A. 1991 I.I. Rabi Award. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5 T. J Magn Reson Imaging. May-Jun 1991;1(3):275-83.
2. Bush AM, Coates TD, Wood JC. Diminished cerebral oxygen extraction and metabolic rate in sickle cell disease using T2 relaxation under spin tagging MRI. Magnetic Resonance in Medicine. 2017;doi:10.1002/mrm.27015
3. Krishnamurthy LC, USA UoTSMCAIRCDT, USA UoTaADoBEAT, et al. Vessel‐specific quantification of blood oxygenation with T2‐relaxation‐under‐phase‐contrast MRI. Magnetic Resonance in Medicine. 2016;71(3):978-989. doi:10.1002/mrm.24750
4. Martin Uecker FO, Jonathan Tamir, Dara Bahri, Patrick Virtue, Joseph Cheng, Tao Zhang, Michael Lustig. Berkeley Advanced Reconstruction Toolbox. Proceedings International Society of Magnetic Resonance in Medicine2015.
Figures
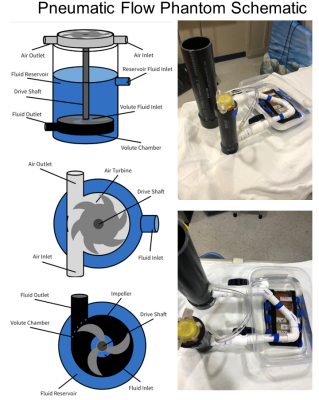
Figure 1: Left) Illustrative representation of the pneumatic flow phantom. Right) Picture of flow phantom used to make T2* estimates.
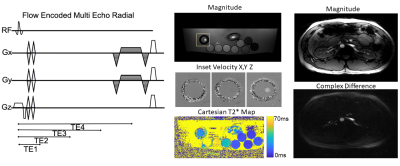
Figure 2: Pulse sequence diagram of the PALMS sequence. Middle) PALMS magnitude and velocity images in phantom and corresponding Cartesian T2* map. Right) Free-breathing PALMS images in the abdomen of a healthy subject.
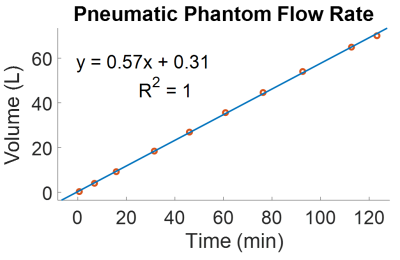
Figure 3: The pneumatic flow phantom generated constant flow over multiple hours as measured by an inline flowmeter.
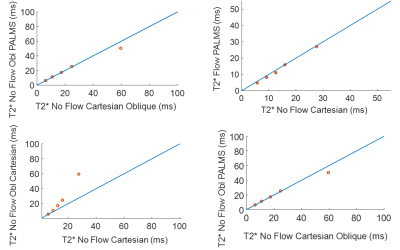
Figure 4: (Top left) Cartesian T2* estimates were inaccurate during the presence of flow (Top Right) however flowing PALMS T2* values matched the non-flowing Cartesian values. (Bottom Left) Vessel obliquity also resulted in inaccurate T2* values, (Bottom Right) however oblique values were comparable between PALMS and Cartesian estimates.