1781
An MRI protocol for RT planning: preliminary results for distortion, contrast and bulk density assignment1Univ Lyon, INSA‐Lyon, Université Claude Bernard Lyon 1, UJM-Saint Etienne, CNRS, Inserm, CREATIS UMR 5220, U1294, F‐69100, Lyon, France, 2CRLCC Léon Bérard – Département de Radiothérapie, Lyon, France, 3CRLCC Léon Bérard – Département de Radiologie, Lyon, France
Synopsis
Keywords: Quantitative Imaging, Head & Neck/ENT, Multimodal
The excellent contrast between soft tissues in MRI is of great interest for head and neck radiotherapy planning. This work presents an in vivo study aiming to evaluate a multimodal acquisition protocol for tumors and organs at risks delineation and synthetic CT reconstruction based on a density assignment method. Our results showed better contrast between the tumor and healthy tissues with injected T1 MRI compared to injected CT (44,7 +/- 30,5 vs 29,9 +/- 27,2) and acceptable geometric distortions (less than 2mm). Fat content mean absolute error between CT and synthetic CT is about 0,27.Introduction
In a context where treatment personalization is becoming necessary, magnetic resonance imaging appears to be a better solution for planification in radiotherapy, due to its capacity to produce high-contrasted images, a better organ at risk (OAR) delineation and quantitative information on the tissues. However, unlike CT scan, MRI does not give direct access to the electronic densities. Two families of approaches allow to build a synthetic CT (sCT) to get through this difficulty: (1) statistical methods like bulk density assignment1, atlas deformation2, or deep learning3; (2) physical methods like proton density4.Our study focuses on the head and neck tumors. This area has not been clinically validated, unlike brain and prostate. It is an interesting but challenging area because of the numerous OARs. This paper describes the phantom developed to quantify the geometric distortions of the multiparametric MRI protocol set to generate a sCT with a bulk density method.
Methods
To quantify the distortions induced by the large field of view, based on the work of Price et al5, we created a distortion phantom composed of several 3D-printed rectangular pieces of 200 x 200 mm with 40 mm height assembled together. 12 mm diameter balls are evenly disposed every 40 mm on the pieces to create a 3D grid with known positions. A total of 19 pieces was necessary to obtain a phantom of 24cm high roughly reproducing the human upper body (Figure 1).A clinical study including 19 patients (5 women, 14 men between 39 and 76 years old), with oropharynx, nasopharynx and larynx tumors was conducted at the comprehensive cancer center Leon Bérard. The 22 minutes MRI acquisition protocol included usual clinical sequences needed for tumors and OARs delineation and two additional sequences for density assignment. It was performed on a 3T Siemens Vida, with 10-15 minutes of installation/uninstallation. The patient was maintained in position with a contention mask used in radiotherapy and dedicated to the patient (Figure 2). Because the head coil could not be used with the mask, the body flex (18 channels), the flex (4 channels) and the spine (14 channels) coils were chosen. The protocol also included a CT scan.
The contrast was computed with the formula $$C= \frac{|I_{lesion} - I_{tissu}|}{I_{lesion} + I_{tissu}}$$ with $$$I_{lesion}$$$ the mean intensity of the lesion area and $$$I_{tissu}$$$ the mean intensity of the healthy area surrounding the lesion.
The bulk density assignment method consists in segmenting the image into different classes of tissues to which electronic densities are assigned. We define four classes corresponding to the fundamental radiological densities: bone, air, water and fat. To distinguish bone and air, we used a 3D Spiral Vibe UTE dual echo with an ultra-short time echo (30µs) and a longer one (4,2ms). Their subtraction allows cortical bone and soft tissues separation; whereas a thresholding of the second echo isolates the air. To discriminate the tissues containing water and fat, we used a 3D Dixon VIBE sequence with two echoes to compute the Proton Density Fat Fraction (PDFF). The mean absolute error (MAE) between sCT and CT fat contents is computed with the formula $$MAE = Σ_{fat content}|I_{MRI} - I_{CT}|$$ with $$$I_{mod}$$$ the pixel intensity for each imaging modality.
Results
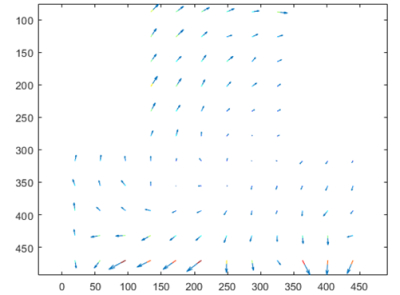
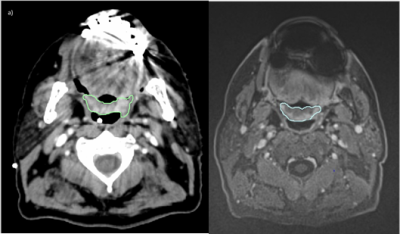
The distortion phantom showed a mean shift of 1,6 +/- 2,6 mm. The center of the field of view benefits from low shifts while the edges show large distortions. Figure 3 shows arrow maps of the shifts.MR injected T1 and injected CT images showed average contrasts of 44,7 +/- 30,5 and 29,9 +/- 27,2 respectively. Nine CT scans were impacted by dental leads artifacts in the region of interest (ROI), see Figure 4, while four MRI exams were impacted in the ROI by artifacts due to swallowing motions. No MRI exam was impacted by dental leads artifacts in the ROI.
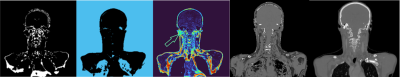
Figure 5 shows the segmentation masks for the 4 classes of densities.
The bone segmentation was difficult due to magnetic susceptibility artifacts. The bone mask obtained here is the product of post treatments like active contours and gradients. The MAE between the normalized PDFF and the normalized reference CT fat content is about 0,27.
Discussion
The mean distortion measured with our phantom is considered acceptable because the voxel size of the planning CT is about 2mm.The protocol allows to benefit from images with a better contrast and less artifacts to build a synthetic CT. However, the computation of the bone mask needs to be improved. We segmented the cortical bone but there are also soft bones (trabecular and marrow) with a different average density. The significant and sudden difference between the magnetic susceptibility of air (~0) and soft tissues (~10-5) induces a decay of T2*, which makes it difficult to separate the bone from the skin, inducing misclassification errors. We currently work on the mapping of the T2* with a multiple-echoes chemical shift encoded sequence to minimize the errors of classification.
Conclusion
Our work demonstrated that MRI could improve dose planning for head and neck tumors by providing a better contrast. However, further works are mandatory to improve bone classification using density assignment method.Acknowledgements
This work was carried out thanks to the financial support of the LABEX PRIMES (ANR-11-LABX-0063) of the University of Lyon, within the framework of the "Investments for the Future" program (ANR-11-IDEX-0007) managed by the National Research Agency (ANR).References
1. Largent « Planification à partir d’imagerie par résonance magnétique en radiothérapie », Cancer/Radiothérapie
2. Johnstone « Systematic Review of Synthetic Computed Tomography Generation Methodologies for Use in Magnetic Resonance Imaging–Only Radiation Therapy » International Journal of Radiation Oncology Biology Physics
3. Vanquin « Planification de la radiothérapie du cancer de la prostate par l’imagerie par résonance magnétique » Cancer/Radiothérapie
4. Kazemifar « Dosimetric evaluation of synthetic CT generated with GANs for MRI‐only proton therapy treatment planning of brain tumors » Journal of Applied Clinical Medical Physics
5. Price « Optimization of a novel large field of view distortion phantom for MR‐only treatment planning » Journal of Applied Clinical Medical Physics
Figures