1779
Zooming In: MR and PET Synaptic Density Imaging Techniques in Neuropathology1Department of Radiology, Yale School of Medicine, New Haven, CT, United States, 2Department of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT, United States, 3Department of Radiology, Norwalk Hospital, Norwalk, CT, United States
Synopsis
Keywords: Quantitative Imaging, PET/MR, Synaptic Density, SV2A
There are many different modalities to image synaptic density in the brain. The advent of a novel SV2A targeted PET tracer provides the ability to measure in-vivo synaptic density changes in neuropsychiatric disorders. In this education exhibit, we review the current MR and PET-based approaches to synaptic density imaging and present findings from recent animal and human studies of synaptic density changes in neurodegenerative disorders and epilepsy. Our aim is to provide clinicians with the most up-to-date knowledge about imaging techniques of synaptic density that can be integrated into clinical practice.INTRODUCTION
Many imaging techniques have been developed to study the structural and molecular changes associated with neuropathology. While MR techniques reveal global structural changes in the brain, there is a critical need to understand dynamics of synaptic density change in neuropathological disorders. The advent of a novel PET tracer targeting synaptic vesicle glycoprotein 2A (SV2A) offers a promising avenue to study in-vivo synaptic density changes1.Educational exhibit objectives:
1. Describe the strengths and limitations of various MR techniques in studying synaptic density
2. Describe the studies that established the correlation between SV2A PET and post-mortem measurement of synaptic density
3. Compare the different properties of 11C and 18F-labeled SV2A tracers
4. Describe the correlation between MR and PET techniques in studying synaptic density
5. Review the published literature on findings of SV2A PET imaging in neurodegenerative disorders and epilepsy
6. Propose the future applications of SV2A PET tracers in neurodegenerative disease and epilepsy imaging.
METHODS
Literature review was conducted for the following topics:1. MR techniques in synaptic density imaging
2. Development and types of SV2A PET tracers
3. Correlation between MR and PET techniques for synaptic density imaging
4. Animal/human studies of SV2A PET imaging in neurodegenerative disorders and epilepsy.
RESULTS
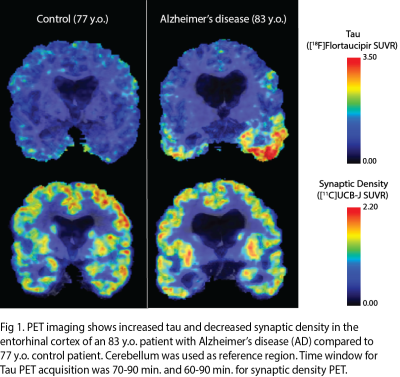
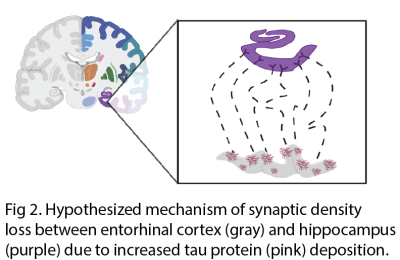
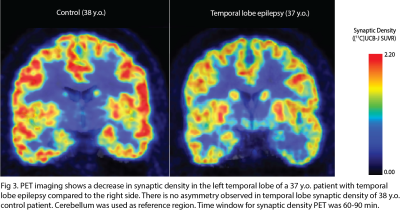
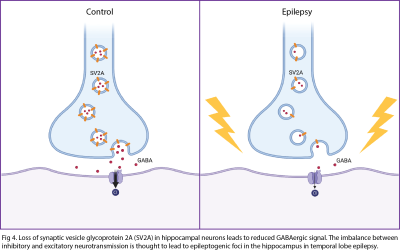
There are various MR techniques to study synaptic density changes in neuropathology. For example, volumetric MRI reveals changes in cortical thickness and brain morphometry while MR spectroscopy studies metabolite concentrations. A new MR technique known as glutamate chemical exchange saturation transfer (GluCEST) spatially maps synaptic changes based on glutamate concentrations. GluCEST shows good correlation with synaptophysin, a synaptic protein marker, in the rat spinocerebellar mossy fiber terminals2. Neurite orientation dispersion and density imaging (NODDI) is a diffusion MRI technique that reveals the complexity of neurites in brain tissue3. In-vivo PET imaging of synaptic density using SV2A tracer is a rapidly burgeoning field. SV2A is a synaptic protein found ubiquitously in the brain and demonstrates excellent correlation with in-vitro SV2A protein levels in primate brain tissue via immunohistochemistry (IHC) and western blotting. The novel [18F] SynVesT-1 SV2A tracer has many advantages over the original [11C] UCB-J SV2A PET tracer, including its larger binding potential (BPND) and longer half-life of isotope4.MR and PET techniques for measuring synaptic density have been correlated in studies of neuropsychiatric disorders. One study revealed a positive correlation between [11C] UCB-J SV2A uptake and glutamate levels measured by MRS in the anterior cingulate cortex (ACC) of healthy volunteers but no correlation between the two measures of synaptic density in patients with schizophrenia5. Another study revealed a significant negative correlation between [11C] UCB-J SV2A density in the dorsolateral prefrontal cortex (dlPFC) and dlPFC-posterior cingulate cortex (PCC) connectivity on fMRI in patients with MDD and PTSD6. Both animal and human studies reveal a significant decrease in SV2A uptake in the hippocampus of patients with Alzheimer’s7–12. SV2A imaging of epilepsy rat models have found that SV2A loss can lead to development of epileptogenic foci and drug resistance due to a decrease in GABAergic inhibitory signals13. Human studies of patients with temporal lobe epilepsy have demonstrated a significant decrease in SV2A uptake in the ipsilateral mesial temporal lobe compared to control patients14.
DISCUSSION
Our educational exhibit discusses the various in-vivo MR techniques used to study synaptic changes in neuropathology. Typical MR techniques, such as volumetric MRI and MRS, assess the structural and molecular changes associated with various neuropathology. While GluCEST MRI has been used as a proxy for studying synaptic density, it is limited to excitatory synapses and has low specificity as studies have shown that up to 30% of GluCEST signal comes from non-glutamate metabolites, including creatine, GABA, and other molecules.Thus, PET imaging using SV2A tracer offers a more direct in-vivo approach to study synaptic density changes. Since SV2A is a ubiquitous protein on presynaptic vesicles in the brain, SV2A PET has more potential than GluCEST in directly studying synaptic density changes. Interestingly, studies of neuropsychiatric disorders have revealed a lack of correlation between SV2A PET and GluCEST in neuropsychiatric disorders, such as schizophrenia, that primarily affect glutamatergic synapses. This further corroborates the strength of SV2A PET over GluCEST MRI in imaging synaptic density in neuropathology.
However, there is also evidence for a combined MR and PET approach to studying synaptic changes in neuropathology. One study uses both fMRI and SV2A PET to reveal how synaptic dysfunction and loss in dlPFC of patients with MDD/PTSD may lead to interconnectivity between dlPFC and PCC, which are typically in opposition to each other to separate internal and external thoughts in healthy patients. Finally, findings from human/animal studies propose future applications of SV2A PET imaging in neurodegenerative disorders and epilepsy, including early detection of Alzheimer’s, treatment response assessment of new Alzheimer’s drugs, and pre-surgical identification of seizure onset zone in epilepsy.
CONCLUSION
While many techniques exist to study synaptic density in neuropathology, each techniques poses strengths and limitations. MR techniques reveal more global structural changes in the brain while PET imaging offers a more granular view of synaptic density changes in neuropathology. Ultimately, a combined MR and PET approach holds the greatest promise for unraveling the mechanisms of pathology in neurological and neuropsychiatric disorders.Acknowledgements
No acknowledgement found.References
1. Cai Z, Li S, Matuskey D, Nabulsi N, Huang Y. PET imaging of synaptic density: A new tool for investigation of neuropsychiatric diseases. Neurosci Lett. 2019;691:44-50. doi:10.1016/j.neulet.2018.07.038
2. Serrano ME, Kim E, Petrinovic MM, Turkheimer F, Cash D. Imaging Synaptic Density: The Next Holy Grail of Neuroscience? Front Neurosci. 2022;16:796129. doi:10.3389/fnins.2022.796129
3. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61(4):1000-1016. doi:10.1016/j.neuroimage.2012.03.072
4. Chen MK, Mecca AP, Naganawa M, et al. Comparison of [11C]UCB-J and [18F]FDG PET in Alzheimer’s disease: A tracer kinetic modeling study. J Cereb Blood Flow Metab. 2021;41(9):2395-2409. doi:10.1177/0271678X211004312
5. Onwordi EC, Whitehurst T, Mansur A, et al. The relationship between synaptic density marker SV2A, glutamate and N-acetyl aspartate levels in healthy volunteers and schizophrenia: a multimodal PET and magnetic resonance spectroscopy brain imaging study. Transl Psychiatry. 2021;11(1):393. doi:10.1038/s41398-021-01515-3
6. Holmes SE, Scheinost D, Finnema SJ, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019;10(1):1529. doi:10.1038/s41467-019-09562-7
7. Colom-Cadena M, Spires-Jones T, Zetterberg H, et al. The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimers Res Ther. 2020;12(1):21. doi:10.1186/s13195-020-00588-4
8. Kong Y, Zhang S, Huang L, et al. Positron Emission Computed Tomography Imaging of Synaptic Vesicle Glycoprotein 2A in Alzheimer’s Disease. Front Aging Neurosci. 2021;13:731114. doi:10.3389/fnagi.2021.731114
9. Mecca AP, O’Dell RS, Sharp ES, et al. Synaptic density and cognitive performance in Alzheimer’s disease: A PET imaging study with [11 C]UCB-J. Alzheimers Dement. Published online February 17, 2022. doi:10.1002/alz.12582
10. Mecca AP, Chen MK, O’Dell RS, et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 2020;16(7):974-982. doi:10.1002/alz.12097
11. Mecca AP, Chen MK, O’Dell RS, et al. Association of entorhinal cortical tau deposition and hippocampal synaptic density in older individuals with normal cognition and early Alzheimer’s disease. Neurobiol Aging. 2022;111:44-53. doi:10.1016/j.neurobiolaging.2021.11.004
12. Toyonaga T, Smith LM, Finnema SJ, et al. In Vivo Synaptic Density Imaging with 11C-UCB-J Detects Treatment Effects of Saracatinib in a Mouse Model of Alzheimer Disease. J Nucl Med. 2019;60(12):1780-1786. doi:10.2967/jnumed.118.223867
13. Mikkelsen JD, Aripaka SS, Bascuñana P, Bankstahl M, Bankstahl JP, Pazarlar BA. Spatio-Temporal Alterations in Synaptic Density During Epileptogenesis in the Rat Brain. Neuroscience. 2022;499:142-151. doi:10.1016/j.neuroscience.2022.07.020
14. Finnema SJ, Toyonaga T, Detyniecki K, et al. Reduced synaptic vesicle protein 2A binding in temporal lobe epilepsy: A [11 C]UCB-J positron emission tomography study. Epilepsia. 2020;61(10):2183-2193. doi:10.1111/epi.16653
Figures