1773
Improved T1w Imaging at Ultra-Low Field by use of phase-sensitive reconstruction1Perinatal Imaging & Health, King's College, London, United Kingdom, 2Hyperfine, London, United Kingdom, 3Department of Neuroimaging, King's College, London, United Kingdom, 4Department of Medical Radiation Physics, Lund University, Lund, Sweden
Synopsis
Keywords: Low-Field MRI, Brain
A phase-sensitive reconstruction is used on adult and neonatal T1w acquisitions on a ultra-low field point-of-care MRI system. We show improved image contrast and reduction in confusing intensities associated with rectification of negative signals.
Introduction
T1w imaging is a critical component of brain MRI. At ultra-low field, where signal to noise ratio (SNR) is low, use of inversion recovery sequences to maximise signal differences is critical, and optimal contrast can often occur when some tissues are inverted and other are not. Magnitude reconstruction results in rebound artefacts (very dark pixels at interfaces where signs change) and confusing lighter appearance for magnetization that is actually inverted. These challenges are particularly prevalent for neonatal brain MRI, where there is generally less T1 dispersion due to longer relaxation times of immature brain tissue [1], and variation of T1 with maturity is a key marker to observe.Another challenge in low SNR regimes is Rician bias in magnitude images, which can obscure subtle differences in low signal areas and causes problems with image combination and in model fitting, such as in relaxometry.
Given this context, there is potential for gains from using a phase-sensitive reconstruction [2] that allows signs to be preserved and avoids both noise bias and rectification artefacts. This approach is applied here in an example adult and neonate to demonstrate its utility.
Method & Materials
Imaging was performed on a portable MRI system operating at 64mT (Hyperfine Swoop, hardware version 1.7, software version 8.5.0) utilising a single transmit/ eight-channel receive head coil. A single adult volunteer was scanned with the manufacturer’s standard “T1w – Grey/White” (TR=1.5s, TI=300ms) and “T1w – Standard” (TR=0.88s, TI=354ms) acquisitions utilising a MR-IR-TSE with the following additional sequence parameters: FOV=220x180x200mm, res=1.6x1.6x5mm, TE=5.84ms, BW=64kHz, TurboFactor=24. A single neonate (gestational age: 33weeks; age at scan: 35weeks) was scanned with multiple MR-IR-TSE sequences with varying TI with the following sequence parameters: FOV=220x180x180mm, res=2x2x2mm, TR=1.5s, TI=[300, 400, 500, 600]ms, TE=7.49ms, BW=64kHz, TurboFactor=48. Standard system reconstructions and also raw data, post electromagnetic interference removal [3], were exported. Separate uncombined images were reconstructed for each receive channel and for even/odd echoes using BART‘s non-uniform FFT [6]. Root sum-of-squares (RSOS) images were created by taking the RSOS across coils for the even and odd echoes separately. These were then registered together using FSL FNIRT [7] and averaged to produce the final RSOS output image.Phase sensitive reconstructions were performed as follows. First receive maps of each coil (Si) were calculated from the longest TI dataset in both the adult and neonatal acquisitions, dividing the image from each receiver (Pj) with the root sum-of-squares (RSOS) combination and applying a median filter of size (7x7). Then a Roemer optimal combination was performed [8] as given by equation 1.
Equation 1
Finally, the even and odd echoes were again aligned using non-rigid registration and averaged to produce the final output (Real Recon in figures 1 and 2).
Results
Figure 1 shows the results of the adult data. The top row shows two example slices with a grayscale chosen with a minimum at zero intensity. The standard reconstruction and real reconstruction have superior SNR, with the RSOS demonstrating high noise levels. The standard and real reconstruction differ in the CSF spaces, where the standard reconstruction exhibits non-zero intensity and within the CSF with a dark (rebound) band indicating signal cancellation due to partial volume effects. This is missing from the real reconstruction. The bottom row shows the same data windows to include the negative values present in the real reconstructions.Figure 2 demonstrates the three reconstructions applied to the neonatal dataset. The images corresponding to TI=300ms and TI=400ms demonstrate improved T1w contrast without the rebound artifacts present on the magnitude reconstructions.
Discussion
In this abstract we have shown the usefulness of complex data for T1w images at ULF. It provides improved contrast depiction with less confusing intensities where there are sign changes. This approach will likely offer benefits to routine weighted imaging (T1w, T2, diffusion and FLAIR) in addition to quantitative methods where Gaussian noise offers benefits to model-fitting. The real reconstruction will also be of use for volumetry, where a sharper boundary is seen between the cortex and CSF. Furthermore, this approach lends itself to acquisitions which require signal averaging.This work has used a dedicated sequence to estimate the receive fields, however this may be unnecessary as coil loading effects are likely to be limited at this field strength, so that a pre-determined coil sensitivity maps could feasibly be used for all reconstructions.
Acknowledgements
This work is supported by the Bill and Melinda Gates Foundation, the MRC (Translation Support Award: MR/V036874/1), the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z], and the Medical Research Council Centre for Neurodevelopmental Disorders [MR/N026063/1].References
[1] Padormo F, Cawley P, et al. In-Vivo T1 mapping of Neonatal Brain Tissue at 64mT. MRM (2022) doi: 10.1002/mrm.29509.
[2] Park HW et al. MRM (1986) doi: 10.1002/mrm.1910030104
[3] Rearick T, Charvat G, Rosen MS, Rothberg J. Noise Suppression Methods and Apparatus. US Patent 9,797,971. March 10, 2016
[4] BART Toolbox for Computational Magnetic Resonance Imaging, DOI: 10.5281/zenodo.592960
[5] Andersson JLR, Jenkinson M, Smith S (2010) Non-linear registration, aka spatial normalisation. FMRIB technical report TR07JA2
[6] Roemer P B, Edelstein W A, Hayes C E, Souza S P, Mueller O M. The NMR phased array. Magnetic Resonance in Medicine (1990). doi:10.1002/mrm.1910160203
Figures
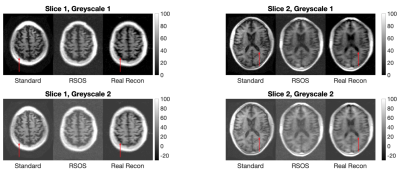
Comparison between standard, RSOS and our real reconstruction for two slices in adult brain and different intensity windowing. Red arrows indicate locations with artifactual CSF signal in standard reconstructions which are corrected by real reconstructions.
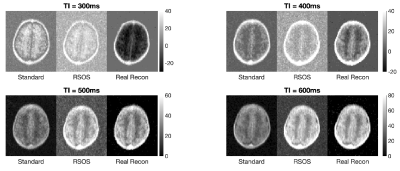
Comparison between standard reconstruction, RSOS and real reconstruction for TI = [300;400;500;600]ms on neonatal brain. The T1 weighting evolves with TI, where inverted tissues at 300ms are negative in real reconstruction then nulled at 400ms, to be entirely positive at 500ms and 600ms.