1772
Breast and Chest RF Design and Optimization for Ultra-low Field MRI
Torben P.P Hornung1,2,3, Neha Koonjoo1,2, Susu Yan3,4, Matthew S Rosen1,2,5, and Thomas R Bortfeld3,4
1Department of Radiology, A.A Martinos Center for Biomedical Imaging / MGH, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Department of Physics, ETH Zürich, Zürich, Switzerland, 4Department of Radiation Oncology, MGH, Boston, MA, United States, 5Department of Physics, Harvard University, Cambridge, MA, United States
1Department of Radiology, A.A Martinos Center for Biomedical Imaging / MGH, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Department of Physics, ETH Zürich, Zürich, Switzerland, 4Department of Radiation Oncology, MGH, Boston, MA, United States, 5Department of Physics, Harvard University, Cambridge, MA, United States
Synopsis
Keywords: Low-Field MRI, Breast, Hybrid & Novel System Technology
MRI guidance in Proton Therapy can improve breast cancer targeting accuracy during treatment planning. This study focuses on RF coil optimization for breast imaging at ultra-low field where proton beam deflection is minimal. Our previous optimization algorithm was further improved to adapt to any breast-shaped coil and any anatomical region. It was also developed for organs-at-risk imaging with a deep view into the chest. This new optimization method was tested for a single-channel conical-shaped coil and validated with imaging. The optimized coil showed a more homogenous B-field compared to its unoptimized evenly spaced wound coil in agreement with the simulations.Introduction
Real-time Ultra-Low Field (ULF) MRI guidance can significantly improve the targeting accuracy of proton therapy (PT) for breast cancer patients. As an initial step in the development of this hybrid system, our aim was to optimize a single-breast RF volume coil on an ULF scanner at 6.5 mT (276.18 kHz). Since treatment planning in RT relies heavily on the sparing of Organs at Risk (OAR), the visibility of the heart is one crucial aspect of MR guided PT of breast tumors. We describe here a new optimization method of a close fitting one-channel RF coil for breast as well as chest wall imaging. Close fitting volume coils are the most suitable at ULF, due to the better filling factor and thus higher signal-to-noise ratio (SNR)1,2. While the SNR conditions exert strict restrictions on the coil shape, the coil winding pattern was used for optimizing simultaneously the magnetic field homogeneity of the coil, the SNR and the magnetic field fall-off into the chest wall. This optimization method can be applied to any breast coil shape. Finally, two breast coils with two different winding patterns were tested and evaluated through imaging: the first coil had an evenly spaced winding pattern (unoptimized coil) and the second one had a more specific winding pattern for a more homogenous B1 field inside the breast and a stronger signal in the chest wall (optimized coil).Materials and Methods
Coil optimization: The coil winding pattern optimization method consists of two consecutive parts. The first optimization step employs Finite Element Method (FEM) simulations (Ansys Maxwell, 2021, Ansys, Canonsburg, PA, USA) as well as Integer Linear Programming and is based on past work by Shen et al.2. It determines for a given coil shape for any inhomogeneity level of the magnetic field in the breast volume and for any magnetic field fall off into the chest wall the minimum wire length needed and thus the maximum possible SNR. It thereby also determines if such a combination of inhomogeneity and magnetic field fall off is feasible for the given coil geometry. The second step of the optimization method consists of an algorithm that delivers the optimal combination of SNR, magnetic field homogeneity and magnetic field fall off into the chest wall for any specified goal. To find this optimal combination, the algorithm defines a solution space of (magnetic field homogeneity in the breast volume, magnetic field fall off in the chest wall) pairs by cutting off unachievable and undesirable pairs. For a selected SNR the algorithm then finds the optimal combination as tangential intersection of the resulting SNR band with an empirically (and for a specific aim) defined iso desire line which represents the trade-off between magnetic field homogeneity inside the breast and magnetic field fall off in the chest wall (Figure 1).Coil description: Our optimization method was applied to two differently shaped (hemispherical, conical) close fitting RF volume coils for breast- and chest wall imaging, but for the sake of this abstract, only the conical-shaped coil is shown (Figure 2). An unoptimized and an optimized conical RF coil were designed using CAD software (Fusion 360, Autodesk, San Rafael, CA, USA). The size of the coils was adapted to the average breast size of American Women. Since the constructed breast coils are all Johnson-Noise dominated, we used stranded Litz wire (Type 1 5/39/42, New England Wire Technologies, NH, USA) to minimize resistive losses. All coils were tuned to 276.18 kHz and matched to at least -40 dB. The loaded Q values were 70 for the un-optimized coil and 65 for the optimized one.
Imaging: A conical water-filled breast-shaped phantom with an internal serpentine structure and a 4 cm deep chest wall region was constructed. This phantom was used for the imaging with a 3D bSSFP sequence and a voxel size of 2.5×3.5×8.5mm3.
Results
The images obtained with the optimized coil are more homogeneous and have a higher signal in the chest wall part of the phantom compared to the images obtained with the unoptimized coil. The optimized coil enables to see the back side of the breast phantom. By increasing the homogeneity and the chest wall signal, the SNR of the images acquired with the optimized coil (76 for 20-averages) fell slightly below the SNR of the images acquired with the unoptimized coil (83 for 20-averages). Figure 3 illustrates 7 image slices of the conical breast phantom obtained with the optimized coil compared to the same 7 image slices of the same phantom acquired with the unoptimized coil.Discussion and Conclusion
This new optimization method was applied to achieve a higher image homogeneity in the breast and an increased view range inside the chest wall in order to meet the image requirements for MR-guided proton therapy. Both goals could be achieved without significantly reducing the SNR. Conclusion: We introduced a new breast coil optimization method and demonstrated its functionality on the example of designing and constructing a conically shaped breast coil for the use in proton therapy. The presented optimization method can be used to design breast coils that meet a wide range of chosen image requirements.Acknowledgements
This work was supported in part by NIH R21CA267315-01A1. MSR acknowledges support of the Kiyomi and Ed Baird MGH Research Scholar award.References
1. M. Sarracanie et al., “Low-cost high-performance MRI,” Sci. Rep.,vol.5,no. 1, Oct. 2015, Art. no. 15177;
2. Shen, S., Xu, Z., Koonjoo, N., & Rosen, M. S. (2020). Optimization of a Close-Fitting Volume RF Coil for Brain Imaging at 6.5 mT Using Linear Programming. IEEE Transactions on Biomedical Engineering, 68(4), 1106-1114.
Figures
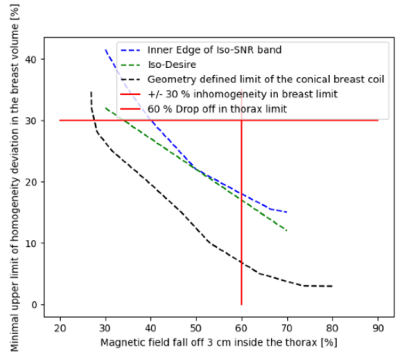
Figure 1: Solution space is restricted by inhomogeneity and B-field fall off limits (red lines) as well as geometric feasibility (black dashed line). Optimal spot is given as tangential intersection of Iso-Desire line (green-dashed line) and inner SNR band edge (blue-dashed line).
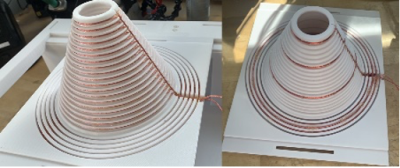
Figure 2: Photographs of the unoptimized (left) and the optimized (right) 1-channel conical breast coils
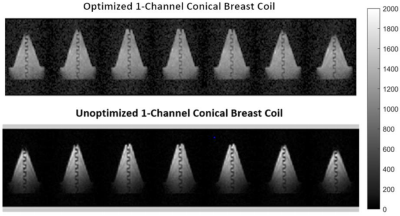
Figure 3: b-SSFP images of the phantom acquired with the optimized (top) and the unoptimized (bottom) 1-channel conical breast coil.
The phantom consists of a conical breast part and a 4 cm long chest-wall part and contains a
serpentine structure. Chosen parameters are - Acquisition time=8min 35sec, NA=20, voxel size=2.5×3.5×8.5mm3. The images obtained with the optimized coil are more homogeneous and show a much stronger signal in the chest-wall
part of the phantom as desired.
DOI: https://doi.org/10.58530/2023/1772