1768
An initial exploration of spiral imaging for quantitative fetal T2* mapping on a low-cost low-field MRI scanner1Center for the Developing Brain, School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Biomedical Engineering Department, School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
Keywords: Low-Field MRI, Data Acquisition
Fetal MRI is an important complementary technique to ultrasound to monitor fetal health. Low-field MRI benefits from reduced distortion, increased B1 homogeneity, wider bore sizes for maintained field homogeneity and longer T2*. The latter is particularly beneficial for single-shot techniques as it allows more flexible and longer read-out strategies. This abstract explores, for the first time, spiral read-out for fetal brain T2* relaxometry and offers preliminary quantitative fetal brain T2* results. Next steps include further exploration of flexible read-out strategies such as undersampled interleaved spirals to work towards higher temporal resolution and motion robustness.Introduction
Fetal development is characterised by a captivating complex chain of events. Unexpected changes can lead to major pregnancy complications affecting both the fetus and mother. The incidence of such complications, for example pre-eclampsia, fetal growth restriction, preterm birth and gestational diabetes, is rising with increasing maternal age and Body mass index. Early detection and management is hence crucial to improve fetal and maternal outcomes.
Fetal MRI plays an important complementary role to ultrasound (US) to gain unique insights into life before birth. While US remains the gold-standard, MRI offers increased resolution and ability to study functional and microstructural properties of tissues. T2* relaxometry, related to the oxygenation of tissue via the BOLD effect, has been particularly widely used in fetal MRI with key organs such as the placenta and fetal brain [1-3] showing reduced T2* associated with pathology.
However, unpredictable fetal motion together with geometrical distortion due to tissue-air interfaces and field inhomogeneities make fetal MRI challenging. Single-shot EPI (ssEPI) techniques that effectively freeze motion are widely used for functional imaging such as T2* mapping. Re-emerging low-field scanners are showing potential to mitigate the mentioned challenges. The longer T2* at low-field is particularly beneficial as it in addition increases the dynamic range, especially in later gestation and cases with pathologies.
While the overall signal strength is reduced, the longer T2* allows the use of longer readouts such as EPI-alternatives including echo-planar time resolved imaging (EPTI) [4] or spiral imaging. Breaking with the rigid EPI regime thereby introduces more flexibility to the sequence, allowing higher temporal resolution as well as sampling the center of k-space more often, potentially helping with the challenge of fetal motion.
Low-field spiral imaging has shown promise on high-performance prototype 0.55T scanners for cardiac imaging, combining motion and flow artifact robustness together with increased signal to noise ratio (SNR) in the myocardium compared to cartesian acquisitions [5].
In this spirit, this study presents, for the first time, a spiral acquisition of the fetal brain in a 0.55T clinical scanner leading to a quantitative T2* map, hence preparing the path for more flexible sampling patterns in the future.
Methods
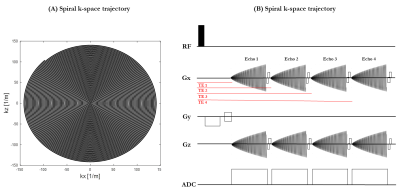
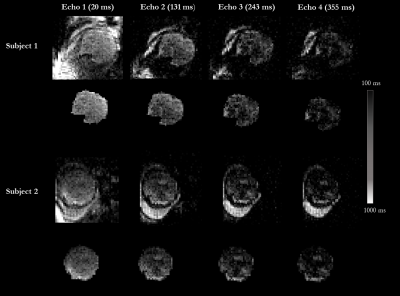
2 healthy pregnant subjects were scanned as part of the ethically-approved Meerkat project (REC 19/LO/0852) on a low-field (0.55T) scanner (MAGNETOM Free.Max, Siemens Healthcare, Erlangen, Germany) in head-first supine position using a 6-channel blanket coil and a 9-element posterior coil with continual life monitoring and verbal interaction. A clinical ssEPI sequence was modified by substituting the EPI readout for a non-cartesian spiral readout and allowing multiple back-to-back readout trains. Multi-echo spiral dataset was acquired in axial and coronal orientation (50 slices, FOV=400x400mm, TR=15950ms, resolution=3.5x3.5x3.5mm and TA=16s). Four different echo times were acquired both in axial orientation (TE=[3,115,226,338]ms) and coronal orientation (TE=[20,131,243,355]ms).As part of a routine research protocol, data was also acquired with a multi-echo ss-EPI sequence (50 slices, FOV= 400x400mm, TR=19170ms, resolution=4x4x4mm, Grappa in-plane acceleration factor 2, TA=28s and TE=[46,120,194,268,342]ms) on the same subjects.
Results
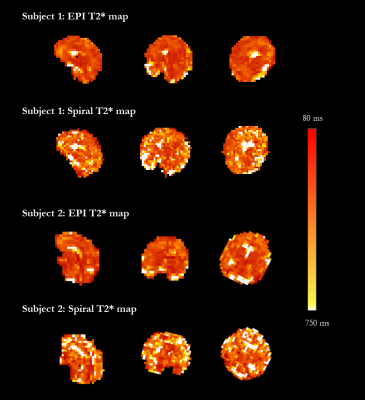
Quantitative T2* maps of the fetal brain were obtained for both EPI and spiral acquisitions, and are displayed in Figure 1 for the scans acquired in the coronal orientation over all TEs.The average T2* brain values for the spiral sequence variant were close to previously reported values for the same gestational age. They were however higher than the matched same subject EPI results, with mean T2* values of around 313ms at 30 weeks GA (second subject) and 301ms at 33 weeks GA (second subject), compared to 274ms and 256ms respectively. Resulting T2* maps for both subjects are shown in Figure 3, illustrating higher noise for the spiral images. Calculated fetal brain volumes were 239ml at 30 weeks and 343ml at 33 weeks for spiral images while EPI imaging ones were 265ml and 254ml, respectively.
Conclusions & Discussion
This first exploration shows the ability to use spiral trajectories on a clinical low-field scanner, most notably with a slew rate of 40mT/m/ms compared to 200mT/m/ms for higher-specification low-field scanners recently presented [5]. The resulting longer spiral duration to achieve the same field-of-view currently limits the resolution and achievable number of subsequent echos.In addition, as shown in the results, reported T2* values are higher than EPI results for the same gestational age which could be a consequence of general noise across the image.
Despite these challenges, the here presented exploration of a more flexible readout opens new possibilities such as exploring interleaved subsampled spiral trajectories, relying less on high slew rates while allowing higher temporal resolution. Furthermore, image quality could benefit from correction of the k-space trajectories prior to reconstruction to account for gradient imperfections [6-7].
Further efforts will focus on improving the current reconstruction pipeline and exploring new spiral trajectory variants. While presented here in the context of T2* mapping, other widely used fetal contrasts such as diffusion MRI or inversion-recovery sequences for T1 mapping could benefit from more flexible readouts such as spirals.
Acknowledgements
The authors thank all pregnant women and their families for taking part in this study. The authors thank the research midwives and radiographers for their crucial involvement in the acquisition of these datasets. This work was supported by a Wellcome Trust Collaboration in Science grant [WT201526/Z/16/Z], a UKRI FL fellowship [MR/T018119/1] and by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z]. The views presented in this study represent these of the authors and not of Guy's and St Thomas' NHS Foundation Trust.References
[1] Blazejewska AI, Seshamani S, McKown SK, Caucutt JS, Dighe M, Gatenby C, et al. 3D in utero quantification of T2* relaxation times in human fetal brain tissues for age optimized structural and functional MRI. Magnetic Resonance in Medicine 2017; 78:909–16. https://doi.org/10.1002/mrm.26471.
[2] Wedegärtner U, Kooijman H, Andreas T, Beindorff N. T2 and T2* measurements of fetal brain oxygenation during hypoxia with MRI at 3T: correlation with fetal arterial blood oxygen saturation. European 2010.
[3]A. Sørensen, J. Hutter, M. Seed, P. E. Grant, and P. Gowland, ‘T2*-weighted placental MRI: basic research tool or emerging clinical test for placental dysfunction?’, Ultrasound Obstet. Gynecol., vol. 55, no. 3, pp. 293–302, Aug. 2020, doi: 10.1002/uog.20855.
[4] F. Wang et al., “Echo planar time-resolved imaging (EPTI),” Magn. Reson. Med., vol. 81, no. 6, pp. 3599–3615, Jun. 2019. https://doi.org/10.1002/mrm.27673.
[5] Matthew C. Restivo,Rajiv Ramasawmy,W. Patricia Bandettini,Daniel A. Herzka,Adrienne E. Campbell-Washburn. Efficient spiral in-out and EPI balanced steady-state free precession cine imaging using a high-performance 0.55T MRI. Magnetic Resonance in Medicine 2020; 84, pp. 2364-2375, https://doi.org/10.1002/mrm.28278.
[6]Barmet C, De Zanche N, Pruessmann KP. Spatiotemporal magnetic field monitoring for MR. Magn. Reson. Med. 2008;60:187–197 doi: 10.1002/mrm.21603.
[7]Vannesjo SJ, Haeberlin M, Kasper L, et al. Gradient system characterization by impulse response measurements with a dynamic field camera. Magn. Reson. Med. 2013;69:583–593 doi: 10.1002/mrm.24263.
Figures