1757
Perfusion and Time of Exchange Measurements Using BBB-ASL in Gliomas: The Initial Experience1Institute of Biomedical Engineering, Bogazici University, Istanbul, Turkey, 2Fraunhofer Institute for Digital Medicine MEVIS, Bremen, Germany, 3Amsterdam University Medical Center, Amsterdam, Netherlands, 4Fraunhofer Institute for Digital Medicine MEVI, Bremen, Germany, 5mediri GmbH, Heidelberg, Germany, 6Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 7Department of Pathology, Acibadem University, Istanbul, Turkey, 8Department of Neurosurgery, Acibadem University, Istanbul, Turkey, 9Department of Radiology, Acibadem University, Istanbul, Turkey, 10Brain Tumor Research Group, Acibadem University, Istanbul, Turkey
Synopsis
Keywords: Tumors, Arterial spin labelling
Subtle changes in BBB integrity might be missed by contrast-enhanced T1-weighted MRI. Blood-brain barrier arterial spin labeling (BBB-ASL) is a new technique to assess BBB disruptions. In this work, we measured the cerebral blood flow (CBF) and exchange time (Tex) values of the tumor, normal-appearing white matter, and normal-appearing gray matter regions in gliomas using BBB-ASL technique. Our results indicated higher CBF and leakier BBB in contrast-enhanced regions of gliomas than in the normal-appearing GM.Introduction
Blood-brain barrier (BBB) breakdown is commonly observed in high-grade gliomas and brain metastases 1 and is typically assessed with the leakage measurement using a contrast agent like gadolinium DTPA (Gd-DTPA) in T1-weighted (T1w) structural MRI. However, subtle changes in BBB integrity might be missed by contrast-enhanced T1w MRI. Blood-brain barrier arterial spin labeling (BBB-ASL) is a new technique that can be used to assess BBB disruptions non-invasively at a more extensive range. This technique has been shown to provide reproducible values of BBB integrity in healthy volunteers 2, and there are first research applications in neurodegeneration 3. However, the feasibility of BBB-integrity measurement in brain tumors with substantial BBB disruption using BBB-ASL has not been demonstrated yet. The aim of this study was to apply the BBB-ASL sequence to assess BBB integrity in gliomas.Methods
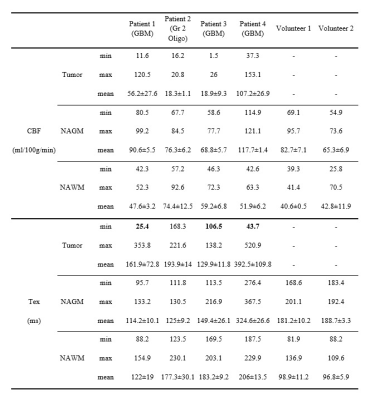
BBB-ASL sequence was used to acquire the MRI data of two healthy volunteers (two females, aged 26 and 44 years) and four histopathologically proven glioma patients (3 glioblastomas (GBM), 1 oligodendroglioma, M/F=3:1, mean age=52.75±14.68 years) using a Siemens 3T MR scanner with a 32-channel head coil. All subjects provided written informed consent in this IRB approved study. Gliomas were graded according to the updated WHO 2021 brain tumor classification. A combination of single-TE and multi-TE Hadamard pseudo-continuous (pCASL) sequences, implemented using the vendor-independent MRI framework gammaSTAR 4 with 3D GRASE readout was used. All measurements were acquired with a voxel size of 5x5x5 mm3. Two measurements of single-TE Hadamard-8 matrix with a sub-bolus duration of 400 ms and a post-labeling delay (PLD [ms]) of 600 and 800, respectively, were acquired (TE= 13.2 ms, TR= 4000 ms, scan time= 02:15 min). The resulting two sets of seven inversion times (TI) [ms] were [1000:400:3400] and [1200:400:3600], respectively. A multi-TE Hadamard-4 matrix with a sub-bolus duration of 1000 ms and a PLD of 500 ms was used. The resulting three TIs [ms] were [1500, 2500, 3500] (TR= 4500 ms, scan time: 01:55 min), and each TI was acquired at eight different echo times (TE [ms]) of [13.8:27.6:207]. Data from the two protocols were concatenated for Tex fitting using FSL FABBER.5 M0 images were acquired twice for geometric distortion correction. Pre- and post- contrast T1-weighted (T1w) SPACE (TR=600 ms, TE=12 ms, slice thickness=0.8 mm) and T2-weighted (T2w) turbo spin echo (TSE) (TR=8880 ms, TE=109 ms, slice thickness=3 mm) MRI data were acquired as structural references. Contrast-enhanced T1-weighted MRI was used to delineate contrast-enhancing regions with strong BBB disruption. Mean CBF and Tex values were calculated within the tumor, normal-appearing white matter (NAWM), and normal-appearing gray matter (NAGM) regions using Slicer software6.Results
Figure 1 shows post-contrast T1w MRI (tumor in green), T2w MRI, CBF, and Tex maps of an example GBM patient. Higher CBF and lower minimum Tex values (except in patient 2 with a grade 2 oligodendroglioma) were observed in contrast-enhancing tumor regions than in NAWM and NAGM (Table 1). While patient 1 and 4 had very high maximum CBF, patient 2, with an oligodendroglioma, had lower CBF values. Although patient 3 had a GBM, he also had a lower maximum CBF due to the localization of the tumor, which was next to the ventricles. Minimum Tex values were lower in the tumor region than NAGM in all GBM patients. Especially, patients 1 and 4 had very low minimum Tex values, indicating highly leaky tumor vasculature. On the other hand, minimum Tex values for patients 2 and 3 were similar to their NAGM values, which might indicate that they had rather intact BBB. The maximum CBF in the tumor region over the mean CBF in NAGM were 1.3, 0.3, 0.4, and 1.3 for these four patients in order, respectively. On the other hand, minimum Tex in tumor region over the mean Tex in NAGM were 0.2, 1.3, 0.7, and 0.1 for these patients.Discussion
In this preliminary study, we have utilized BBB-ASL MRI to calculate perfusion and exchange times of tumor region, white matter, and gray matter in volunteers and gliomas. One limitation of this study was the heterogeneity of Tex values within the tumor region, especially regions of very high Tex in the two GBM patients. Further investigation needs to clarify the effects of tumor-tissue T2-time heterogeneity, which might not be assessable with contrast-enhanced T1w MRI, and its effect on Tex quantification. This pilot study shows the feasibility of BBB-integrity measurement with multi-echo BBB-ASL demonstrating sensitivity to BBB-disruption in contrast-enhancing glioblastomas with increased perfusion. Future studies will acquire BBB-ASL data of more glioma patients for assessing changes in the exchange rate of water across the BBB for grading tumor malignancy and identifying genetic mutational subgroups of gliomas.Acknowledgements
The DEBBIE project (Developing a non-invasive biomarker for early BBB breakdown in Alzheimer's disease) is an EU Joint Programme -Neurodegenerative Disease Research (JPND) project. It is supported through the following funding organisations under the aegis of JPND -www.jpnd.eu (FWO in Belgium, Canadian Institutes of Health Research in Canada, BMBF in Germany, NFR in Norway, ZonMw in The Netherlands, TUBITAK (grant number 121N030) in Turkey). The project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 825664. This publication is also a part of the COST Action CA18206 Glioma MR Imaging 2.0, supported by COST (European Cooperation in Science and Technology.References
1. Arvanitis CD, Ferraro GB, and Jain RK, The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer 2020;20(1):26-41.
2. Mahroo A, Buck MA, Huber J, et al. Robust Multi-TE ASL-Based Blood-Brain Barrier Integrity Measurements. Front Neurosci 2021;15:719676.
3. Ohene Y, Harrison IF, Evans PG, Thomas DL, Lythgoe MF and Wells JA, Increased blood-brain barrier permeability to water in the aging brain detected using noninvasive multi-TE ASL MRI. Magn Reson Med 2021;85(1):326-333.
4. Cordes C, Konstandin S, Porter D, Günther M. Portable and platform‐independent MR pulse sequence programs. Magn Reson Med 2020;83(4):1277–90.
5. Woolrich M, Chiarelli P, Gallichan D, Perthen J, Liu T. Bayesian Inference of Haemodynamic Changes in Functional ASL Data. Magn Reson Med 2006;56:891-906.
6. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 2012;30(9): 1323-1341.