1754
Preoperative ASL correlates with molecular markers of IDH and HIF-1α in glioma microenvironment and is predictive of patient outcome1Radiology, The Second Hospital, Dalian Medical University, Dalian, China, 2GE Healthcare, MR Research China, Beijing, China
Synopsis
Keywords: Tumors, Arterial spin labelling
This study retrospectively analyzed the association between arterial spin labeling (ASL) and the molecular markers of isocitrate-dehydrogenase (IDH) mutation and hypoxia-inducible factor (HIF) -1α in glioma. The association of patients’ overall survival (OS) and progression-free survival (PFS) with quantization cerebral blood flow (CBF) from ASL, the imaging parameters from conventional MRI, clinical and pathological parameters were investigated in glioma.Background or purpose
Glioma are highly infiltrative and resistant to therapy, rendering them largely incurable, regardless of grade. IDH gene mutation has been identified as a favorable prognostic factor. However, the recurrence and poor prognosis of most gliomas have not been fully evaluated. Arterial spin labeling imaging can reflect the grade, angiogenesis and IDH gene mutation status to a certain extent1-3, but its correlation with hypoxic state of glioma has not been fully demonstrated. Hypoxia promotes glioma angiogenesis, stem cell replication and tumor proliferation, which is closely related to the decline of radio chemotherapy sensitivity of glioma4. It is an important factor for prognosis evaluation. We hypothesized that CBF is closely related to the expression of IDH mutation and HIF-1α in glioma. And to explore the value of ASL, conventional MRI, clinical data and pathological parameters in evaluating overall survival (OS) and progression-free survival (PFS) of glioma.Material and Methods
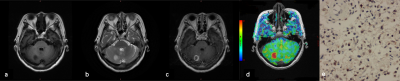
This study retrospectively analyzed the ASL and conventional MR images of sixty-four glioma patients, who underwent maximal surgical resection or biopsy followed by concurrent chemoradiotherapy and adjuvant chemotherapy using temozolomide. The MR examination were scanned at 3.0T MRI (GE Healthcare, 750W). Regions of interest were drawn to obtain cerebral blood flow (CBF). The CBF and relative CBF (rCBF) from ASL, the imaging characteristics from conventional MR images, the clinical data and the related pathological parameters were analyzed. The clinical data included gender, age, surgical method, postoperative treatment, symptoms, KPS score. The image characteristics included the maximal tumor diameter, ventricle invaded, single or multiple shots, boundary, cystic, necrosis, hemorrhage, reinforcement. The glioma tissues were obtained for evaluating pathological grade, the expression of IDH mutation status, MGMT, ARTX, ki-67, HIF-1α. In addition, OS was divided into short-term group and long-term group with 12 months. The differences of related factors between the two groups were compared. Association of patient OS/PFS with image parameters, clinical and pathological parameters were assessed using the univariate Cox proportional hazards regression model. To evaluate the relationship between HIF-1α, IDH1 and ASL parameters, Spearman rank correlation analysis was performed. Chi-square test and Fisher's exact test were applied for frequency tables.Results
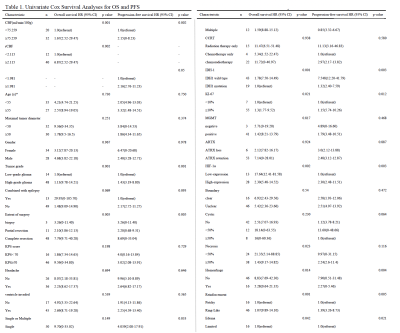
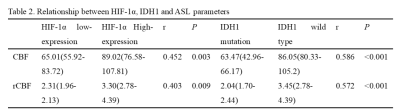
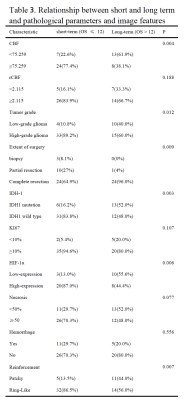
Results of the univariate analyses are shown in table 1 and figure 1. CBF, rCBF, tumor grade, IDH-1 mutated status, Ki-67, HIF-1α, extent of surgery, necrosis, hemorrhage, reinforcement were valuable parameters for OS (P < 0.05). Tumor grade, IDH, Ki-67, HIF-1α, extent of surgery, hemorrhage, reinforcement, edema were valuable parameters for PFS (P < 0.05). There is a significant difference in CBF and rCBF between IDH wild-type and mutant groups. Similarly, there is a significant difference in high and low expression of HIF-1α (Table 2). The further analysis about short-term and long-term OS group were summarized in Table 3. In the patients with CBF higher than 75.259ml/min/100g and rCBF higher than 2.115, there were 77.4% (24/31) and 83.9% (26/40) cases belonged to short-term survival group separately (Fig.2). The following characteristics of patients with short survival period are statistically different, such as high-grade glioma, despite full resection, IDH-wild type, HIF-1α high expression and ring enhancement.Discussion
The evaluation of IDH mutation and hypoxia microenvironment in glioma has very important clinical significance. Previous study has shown that DCE-MR may facilitate noninvasive preoperative predictions of areas of tumor with increased hypoxia and proliferation5.In this study, CBF and rCBF were associated with IDH mutation status and the expression of HIF-1α. The IDH wild-type and the HIF-1α overexpression groups of gliomas had higher CBF.CBF and rCBF are associated with OS and PFS in glioma and are useful parameters for predicting short-term survival. Previous studies using DSC perfusion technology found that the reduction of tissue oxygen tension in glioma, that is, local hypoxia, occurred before the change of micro vessels, is a risk factor for local recurrence6. We use the ASL perfusion method to reach the same conclusion. Besides these, the pathological parameters including tumor grade, IDH-1, Ki-67, HIF-1α expression were also related to prognosis in gliomas, there were representative molecular markers for prognosis information in gliomas. Previous studies have identified that IDH mutation can regulate HIF-1α7, which is a driving force in tumorigenesis and angiogenesis. Therefore, we speculated that the HIF-1α high expression could represent the poor prognosis of glioma. Moreover, the present study found that glioma showed a ring-like enhancement. Tumors with ring-like enhancement may benefit more from gross total surgical resection, which is associated with longer survival in glioma patients.Conclusions
CBF measured by 3D-ASL could be used for non-invasively evaluation the IDH mutation status and the expression of HIF-1α in glioma, and is related to the OS and PFS. Pathological parameters and image features from conventional MR help to predict glioma OS and PFS.Acknowledgements
No acknowledgement found.References
1.Shen, N. et al. Intravoxel incoherent motion diffusion-weighted imaging analysis of diffusion and microperfusion in grading gliomas and comparison with arterial spin labeling for evaluation of tumor perfusion. J Magn Reson Imaging 44, 620-632, doi:10.1002/jmri.25191 (2016).
2.Pang, H. et al. 3D-ASL perfusion correlates with VEGF expression and overall survival in glioma patients: Comparison of quantitative perfusion and pathology on accurate spatial location-matched basis. J Magn Reson Imaging 50, 209-220, doi:10.1002/jmri.26562 (2019).
3.Peng, H. et al. Predicting Isocitrate Dehydrogenase (IDH) Mutation Status in Gliomas Using Multiparameter MRI Radiomics Features. J Magn Reson Imaging 53, 1399-1407, doi:10.1002/jmri.27434 (2021).
4.Shen, H., Cook, K., Gee, H. E. & Hau, E. Hypoxia, metabolism, and the circadian clock: new links to overcome radiation resistance in high-grade gliomas. J Exp Clin Cancer Res 39, 129, doi:10.1186/s13046-020-01639-2 (2020).
5.Jensen, R. L. et al. Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol 16, 280-291, doi:10.1093/neuonc/not148 (2014).
6.Stadlbauer, A. et al. Hypoxia and Microvascular Alterations Are Early Predictors of IDH-Mutated Anaplastic Glioma Recurrence. Cancers (Basel) 13, doi:10.3390/cancers13081797 (2021).
7. Zhao, S. et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science 324, 261-265, doi:10.1126/science.1170944 (2009).
Figures