1751
Intravoxel Tissue Heterogeneity Revealed by Non-Gaussian Time-Dependent Diffusion MRI and Its Correlation with Histology1Center for Magnetic Resonance Research, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Biomedical Engineering, University of Illinois at Chicago, Chicago, IL, United States, 3Research Resource Center, University of Illinois at Chicago, Chicago, IL, United States, 4Department of Radiology, Northwestern University, Evanston, IL, United States, 5Departments of Radiology and Neurosurgery, University of Illinois College of Medicine, Chicago, IL, United States
Synopsis
Keywords: Tumors, Tumor, Glioma
Recently, diffusion-weighted MRI has emerged for probing intravoxel structural heterogeneity non-invasively as an alternative to gold standard histopathological analysis. A previous study demonstrated the correlation between the imaging- and histology-based tissue heterogeneities using a continuous-time random-walk (CTRW) model at a single diffusion time. In this study, we extended the study to multiple diffusion times and investigated the diffusion time dependence of the CTRW parameters and their correlation with histology-based heterogeneity on human glioma tissues. Significant time dependency was observed in the CTRW parameters, providing practical insights into the selection of diffusion time when assessing tissue heterogeneity.Introduction
Tissue heterogeneity has been one of the most important criteria for cancer diagnosis1,2. While histopathological analysis of the excised tissue serves as the gold standard for the assessment of tissue heterogeneity, non-Gaussian diffusion-weighted MRI (DWI) has attracted increasing attention as an alternative approach for probing tissue structural heterogeneity non-invasively3-6. For example, DWI with a continuous-time random-walk (CTRW) model or its variant has illustrated the feasibility for characterizing intravoxel heterogeneity in several cancerous tissues6-9 More recently, a rigorous correlation between imaging-based diffusion heterogeneity revealed by CTRW parameters and histology-based tissue structural heterogeneity was reported based on DWI experiments employing a single diffusion time (Δ).10 However, it has been recognized that the non-Gaussian diffusion process and the parameters derived from diffusion models strongly depend on Δ11,12. In this study, we investigate the CTRW parameters at different Δ’s and their correlation with histology-based heterogeneity level probability maps determined by using machine learning.Methods
Image Acquisition/Analysis: A human postmortem glioma tissue sample (GLs) fixed in paraformaldehyde was scanned on a 9.4T Agilent MRI scanner. DWI datasets with 12 b-values from 0 to 5000s/mm² were obtained using a pulsed-gradient stimulated echo sequence. For the set of b-values, seven different Δ’s (16, 30, 50, 80, 100, 120, and 150ms) were investigated. The other imaging parameters were: TR/TE = 3000/16ms, slice thickness = 0.3mm, in-plane resolution = 0.28×0.28mm2, and δ = 3.0ms. The scan time was 50 hours 45 minutes. Trace-weighted diffusion images were analyzed using a time-dependent CTRW model11,$$S/S0 = E_{\alpha}(-D_{\alpha,\beta}q^{2\beta}\Delta^{\alpha})$$ where Dα,β is anomalous diffusion coefficient, q = γGδ (γ, gyrometric ratio; G, diffusion gradient amplitude; δ, diffusion gradient pulse width), Eα is a Mittag-Leffler function, α and β are temporal and spatial diffusion heterogeneity parameters, respectively. A non-linear least-squares algorithm was used to estimate the CTRW parameters.Histological Analysis: After MRI, the specimen was H&E stained and cut into 5 µm-thick histology sections corresponding to the middle region of each imaging slice. Each digitized section was partitioned into small tiles with the same spatial resolution as the diffusion-weighted images. 33 statistical features were extracted in each tile to characterize structural features related to heterogeneity. After feature extraction, a trained machine learning classifier10 was employed to predict the probability of a given tile to have heterogeneity level of H1, H2, or H3 [$$$p(H1)+p(H2)+p(H3)=1$$$].
Co-registration and Statistical Analysis: Histology-based heterogeneity level probability maps determined by Hi (i=1,2,3) were co-registered to diffusion-weighted images through an affine transformation. Regions-of-interest (ROIs) were drawn over each heterogeneity level on the histology heterogeneity maps. At each heterogeneity level, a one-way ANOVA was employed to examine the significance of differences in the mean CTRW parameter values among the seven Δ values. Pairwise comparisons were performed to investigate statistical differences of CTRW parameters between short (16ms), intermediate (80ms), and long (150ms) diffusion times for all heterogeneity levels. The three CTRW parameters were combined by using a multivariable logistic regression. This was followed by evaluating the performance of the combination of the CTRW parameters in delineating pathological tissue heterogeneity with pairwise comparisons (H1 vs. H2, H2 vs. H3, and H1 vs. H3). Sensitivity, specificity, and AUC were investigated in an ROC analysis.
Results
Figure 1 displays a set of representative Dα,β, α, and β maps at seven Δ’s where all parameters exhibited a decreasing trend as Δ increased. Figures 2a–2c show the co-registered heterogeneity level probability maps of H1, H2, and H3, respectively. Figure 2d illustrates a composite heterogeneity map created by displaying the highest probability of the heterogeneity level at each pixel. The time dependency is illustrated in Figure 3 where the CTRW parameter values within the ROIs of all slices are shown for each Δ. One-way ANOVA analysis yielded statistically significant differences (p<10-4) between the CTRW parameter values obtained at different diffusion times at each heterogeneity level. Again, as Δ increased, all CTRW parameters exhibited a decreasing trend. A significant difference (p<.05) was observed in all pairwise comparisons (16ms vs. 80ms, 16ms vs. 150ms, and 80ms vs. 150ms). Figure 4 summarizes the sensitivity, specificity, and AUC values for delineating different histological heterogeneity levels at different diffusion times using the combination of CTRW parameters. H1 vs. H3 yielded the highest sensitivity (0.874), specificity (0.911), and AUC (0.920).Discussion and Conclusion
In this study, we investigated the diffusion time dependence of the CTRW parameters of human glioma tissues and correlated the time-dependent CTRW parameters with histology-based tissue structural heterogeneity. The decreasing trend observed in all CTRW parameters with an increasing diffusion time is consistent with a previous phantom study11. In general, shorter diffusion times yielded higher AUCs in differentiating the heterogeneity levels, although there were exceptions. Previous reports indicate that Fluorinert immersion has no effect on normal tissue integrity and the associated histological analysis13,14. However, its impact on water diffusion dynamics in glioma tissues have not been well studied. Fluorinert immersion could be a possible explanation on the higher CTRW parameter values observed at higher heterogeneity levels (Figure 2), which is opposite to the observations in previous in vivo and ex vivo studies7,11,12. Nevertheless, this study provides insights for understanding how the diffusion time affects the CTRW parameters and their correlations to tissue heterogeneity derived from histology.Acknowledgements
This work was supported in part by the National Institutes of Health (5R01EB026716-01 and 1S10RR028898-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Drs. Alessandro Scotti and Kezhou Wang for helpful discussions.
References
[1] Bedard PL, Hansen AR, Ratain MJ, et al. Tumour heterogeneity in the clinic. Nature. 2013;501(7467):355–64.
[2] Fletcher CDM. The evolving classification of soft tissue tumors – an update based on the new 2013 WHO classification. Histopathology. 2014;64(1):2-11.
[3] Zhang, A., Hu, Q., Song, J., et al. Value of non-Gaussian diffusion imaging with a fractional order calculus model combined with conventional MRI for differentiating histological types of cervical cancer. Magnetic Resonance Imaging, 2022;93, 181-188.
[4] Suo, S., Chen, X., Wu, L., et al. Non-Gaussian water diffusion kurtosis imaging of prostate cancer. Magnetic Resonance Imaging, 2014;32(5), 421-427.
[5] Szczepankiewicz, F., van Westen, D., Englund, E., et al. The link between diffusion MRI and tumor heterogeneity: Mapping cell eccentricity and density by diffusional variance decomposition (DIVIDE). Neuroimage, 2016;142, 522-532.
[6] Sui, Y., Wang, H., Liu, et al. Differentiation of low-and high-grade pediatric brain tumors with high b-value diffusion-weighted MR imaging and a fractional order calculus model. Radiology, 2015;277(2), 489-496.
[7] Karaman, M. M., Sui, Y., Wang, H., et al. Differentiating low‐and high‐grade pediatric brain tumors using a continuous‐time random‐walk diffusion model at high b‐values. Magnetic resonance in medicine, 2016; 76(4), 1149-1157.
[8] Qin, Y., Tang, C., Hu, Q., et al. Assessment of Prognostic Factors and Molecular Subtypes of Breast Cancer with a Continuous-Time Random-Walk MR Diffusion Model: Using Whole Tumor Histogram Analysis. Journal of Magnetic Resonance Imaging, 2022.
[9] Feng, C., Wang, Y., Dan, G., et al. Evaluation of a fractional-order calculus diffusion model and bi-parametric VI-RADS for staging and grading bladder urothelial carcinoma. European Radiology, 2022;32(2), 890-900.
[10] Karaman, M, Dan, G, Sha L, et al. Looking inside a voxel through the lenses of non-Gaussian diffusion MRI: correlation between imaging- and histology-based tissue heterogeneity. In Proceeding of the 29th Annual Meeting of ISMRM. 2021;0701.
[11] Dan, G., Li, W., Zhong, Z., et al. Diffusion in Sephadex Gel Structures: Time Dependency Revealed by Multi-Sequence Acquisition over a Broad Diffusion Time Range. Mathematics. 2021;9(14), 1688.
[12] Ingo, C., Magin, R. L., Colon‐Perez, L., et al. On random walks and entropy in diffusion‐weighted magnetic resonance imaging studies of neural tissue. Magnetic resonance in medicine, 2014;71(2), 617-627.
[13] Iglesias JE, Crampsie S, Strand C, et al. Effect of Fluorinert on the histological properties of formalin-fixed human brain tissue. J Neuropathol ExpNeurol. 2018; 77(12): 1085–1090.
[14] Hyare H, Powell C, Thornton J, et al. Perfluoropolyethers in magnetic resonance microscopy: Effect on quantitative magnetic resonance imaging measures and histological properties of formalin-fixed brain tissue. In Proceeding of the 16th Annual Meeting of ISMRM. 2008;1719.
Figures
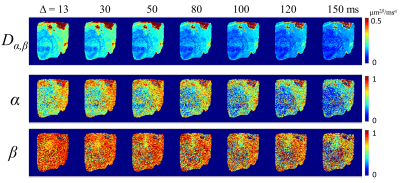
Figure 1. CTRW parameter maps (Dα,β, α, and β) from a representative diffusion MRI slice at different Δ values (13–150ms).
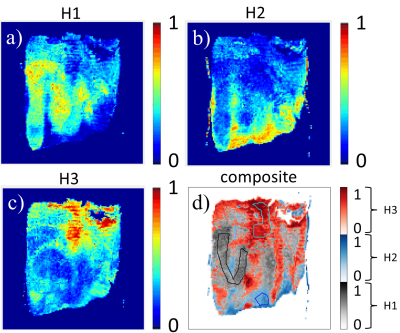
Figure 2. a) – c): Histology-based heterogeneity maps after they were co-registered with the diffusion-weighted images. d): The composite heterogeneity map generated by showing the highest probability of heterogeneity level of each voxel, as indicated by the custom color bar. The ROIs of H1, H2, and H3 are shown in black, blue, and cyan, respectively.
Figure 3. Boxplots of Dα,β, α, and β values against diffusion time within the ROIs of each heterogeneity level (H1, H2, and H3). The p-values generated by one-way ANOVA analysis are labeled in each box plot. Each diffusion time is represented by a distinct color.
Figure 4. The performance of the CTRW parameters in delineating different histology-based heterogeneity levels at different diffusion times.