1750
Healthy and cancerous brain ADC: diffusion- and echo time dependence with pulsed gradient spin-echo (PGSE) diffusion imaging
Jens Johansson1, Kerstin Lagerstrand2,3, Isabella M Björk-Burtscher1,4, Mats Laesser1,5, Hanna Hebelka1,4, and Stephan E Maier1,6
1Radiology, Clinical sciences,Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 2Medical Radiation Sciences, Clinical sciences,Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 3Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden, 4Radiology, Sahlgrenska University Hospital, Gothenburg, Sweden, 5Sahlgrenska University Hospital, Gothenburg, Sweden, 6Radiology, Brigham and women's hospital, Boston, MA, United States
1Radiology, Clinical sciences,Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 2Medical Radiation Sciences, Clinical sciences,Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 3Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden, 4Radiology, Sahlgrenska University Hospital, Gothenburg, Sweden, 5Sahlgrenska University Hospital, Gothenburg, Sweden, 6Radiology, Brigham and women's hospital, Boston, MA, United States
Synopsis
Keywords: Tumors, Diffusion/other diffusion imaging techniques, Diffusion time
Clinical systems vary widely in their gradient performance. Accordingly, effective diffusion time and echo time, which are minimized by the system software according to available gradient performance, can differ significantly among systems. A major concern is that non-Gaussian diffusion in tissues can lead to significant variation of the measured apparent diffusion coefficient (ADC), which would clearly diminish its value as biomarker. This study evaluated if diffusion and/or echo time variations in pulsed gradient spin-echo (PGSE) sequences significantly influences the ADC measured in healthy brain and brain tumors.Introduction
Although the ADC is considered a valuable quantitative biomarker in clinical diffusion-weighted imaging (DWI) 1,2, it has been shown to be dependent on diffusion time (TD), echo time (TE) and b-value choice in both healthy and cancerous brain tissue 3–10. Intra- and extracellular space exhibit different diffusivity and T2-relaxation. Thus, changing the scan parameters can influence the relative signal contribution of respective compartments 11. The application of oscillating gradient spin echo (OGSE) and stimulated echo acquisition mode (STEAM) sequences on clinical systems to achieve very short diffusion times, respectively very long diffusion times, has been reported. In a clinical setting, however, the predominantly used sequence is a pulsed-gradient spin echo (PGSE) sequence. The higher gradient performance available on some state-of-the-art systems can significantly reduce the effective diffusion time and the echo time for PGSE sequences with a given maximum b-value. The present study investigated the effect of varying diffusion time and/or echo time on the ADC measured with a PGSE sequence in healthy brain tissue and brain tumors.Methods
Ten healthy volunteers and ten patients with brain tumors were recruited and scanned with a modified PGSE DWI sequence that independently allowed the control of diffusion time and echo time. Scans were performed at a maximum gradient amplitude of 68 mT/m for effective diffusion times (TD = Δ - δ/3) 16, 40 and 70 ms (with TE = 104 ms) and echo times 60, 75 and 104 ms (with TD = 16 ms) (Fig.1). In an additional scan, diffusion- and echo time were increased simultaneously (TD = 31 ms, TE = 104 ms), thus, simulating a system with low gradient performance of 19 mT/m (Table 1).Sequence parameters common to all scans were TR: 7000 ms; slice thickness/gap: 3.6/0.4 mm; FOV: 240x240 mm; matrix size: 168x128, three orthogonal diffusion directions and b-values of 0, 100 and 1000 s/mm2; multi-coil acceleration: R=2; 3 Tesla scanner (Premier, GE Healthcare, Milwaukee WI, USA) with a 48-channel head coil (Gmax = 80 mT/m, maximum slew rate = 200 mT/m/ms). T2- and T1-weighted images were acquired to aid ROI delineation.
Maps of the ADC were generated from diffusion weightings 0 and 1000 s/mm2 and from 100 and 1000 s/mm2. The ADC was measured in ROIs on an axial map. Healthy brain ROIs were defined for the splenium and genu of the carpus callosum, white matter, and bilaterally in the putamen, caudate nucleus, and thalamus. Brain tumor ROIs were also defined, whereby suspected necrosis or surrounding edema were excluded.
Results
In normal brain tissues, for ADC maps generated with bmin = 100, relative differences occurring with diffusion time change ranged from -3.0% to 3.1%. Similarly, changes associated with echo time and gradient performance ranged from -4.1% to 1.2% and -1.5% to 4.6%, respectively. In some regions, the changes were significant (tested with Friedmans (Tukey-Kramer post-hoc) and Wilcoxon signed-rank test). For comparison, changes occurring with repeated scans performed in 8 subjects ranged from -0.9% to 3.8%. Results for bmin = 0 were similar.The relative difference for brain tumors with diffusion time change ranged from -5.7% to 5.8 %, with echo time from -8.9% to 5.5%, and with gradient performance from -19% to 7.6 %. In most brain tumors, mean ADC increased modestly with diffusion time (Fig. 2a). For tumors with diffusivity above ~1000x10-6 mm2/s, there was a pronounced ADC increase with echo time. Meanwhile, for tumors with diffusivity below ~1000x10-6 mm2/s, there was a minimal change or reduction (Fig. 2b). A similar trend was observed for a reduction in gradient performance (Fig. 2c). All trends were present for both ADCb0,1000 and ADCb100,1000. Example of ADC maps from a patient with diffuse large B-cell lymphoma are shown in Fig. 3.
Discussion
Changes in normal brain tissue are relatively small for the explored range of parameter variation. The absence of significant diffusion time dependence can be explained by the presence of axons, where the diffusion is completely restricted. The absence of any changes related to echo time and gradient performance is less obvious but may be due to transversal relaxation times that are similar for intra-axonal and extracellular water.Changes observed in brain tumors are much more distinct and surpass typical variation of a repeat exam many-fold. This can be a clear impediment to the ubiquitous use of ADC as a quantitative biomarker. There may be a clear relation between echo-time dependent changes and overall diffusivity, but this needs to be verified in a study with a larger sample size. Overall, reporting all relevant scan parameters is important and protocol harmonization is helpful to minimize variation of the quantitative ADC biomarker. It would also be desirable to implement pulse sequences that offer flexibility in choosing effective diffusion time and echo time.
Conclusion
With PGSE sequences the presently accessible range in gradient performance for clinical scanners, signifies a considerable range of echo times, diffusion times and gradient strengths among scanners for a given maximum b-value. The resulting ADC in tumors can be markedly influenced by the variation of these parameters and consideration of this fact may be necessary when conducting multi-center studies or using ADC as biomarker in clinical routine.Acknowledgements
Barncancerfonden
The Swedish state under an agreement between the Swedish government and the country councils (ALFGBG-932648)
The Swedish Research Council (Vetenskapsrådet)
The Swedish Cancer Society (Cancerfonden)
References
1. Baboli M, Zhang J, Kim SG. Advances in Diffusion and Perfusion MRI for Quantitative Cancer Imaging. Curr. Pathobiol. Rep. 2019;7(4):129–141.2. Drake-Pérez M, Boto J, Fitsiori A, et al. Clinical applications of diffusion weighted imaging in neuroradiology. Insights Imaging 2018;9(4):535–547.
3. Baron CA, Beaulieu C. Oscillating gradient spin-echo (OGSE) diffusion tensor imaging of the human brain. Magn. Reson. Med. 2014;72(3):726–736.
4. Tétreault P, Harkins KD, Baron CA, et al. Diffusion time dependency along the human corpus callosum and exploration of age and sex differences as assessed by oscillating gradient spin-echo diffusion tensor imaging. Neuroimage 2020;210.
5. Lin M, He H, Tong Q, et al. Effect of myelin water exchange on DTI-derived parameters in diffusion MRI: Elucidation of TE dependence. Magn. Reson. Med. 2018;79(3):1650–1660.
6. Fieremans E, Burcaw LM, Lee HH, et al. In vivo observation and biophysical interpretation of time-dependent diffusion in human white matter. Neuroimage 2016;129:414–427.
7. Lee HH, Papaioannou A, Novikov DS, et al. In vivo observation and biophysical interpretation of time-dependent diffusion in human cortical gray matter. Neuroimage 2020;222:117054.
8. Clark CA, Hedehus M, Moseley ME. Diffusion time dependence of the apparent diffusion tensor in healthy human brain and white matter disease. Magn. Reson. Med. 2001;45(6):1126–1129.
9. Maekawa T, Hori M, Murata K, et al. Differentiation of high-grade and low-grade intra-axial brain tumors by time-dependent diffusion MRI. Magn. Reson. Imaging 2020;72:34–41.
10. Iima M, Yamamoto A, Kataoka M, et al. Time-dependent diffusion MRI to distinguish malignant from benign head and neck tumors. J. Magn. Reson. Imaging 2019;50(1):88–95.
11. Qin W, Yu CS, Zhang F, et al. Effects of echo time on diffusion quantification of brain white matter at 1.5T and 3.0T. Magn. Reson. Med. 2009;61(4):755–760.
Figures
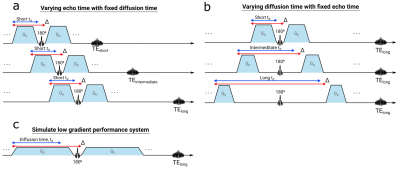
Fig.1 The different scan scenarios to investigate the dependency of (a) echo time, (b) diffusion time (here td) and (c) gradients performance on the measured ADC.
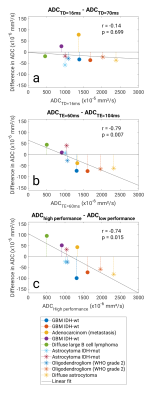
Fig.2 Difference in the mean ADC between a) short/long diffusion time, b) short/long echo time and c) high/low gradient strength for ADC from b = 0 & 1000 s/mm2 (top row) and ADC from b = 100 & 1000 s/mm2 (bottom row). Pearsons correlations coefficient were calculated for each plot and are displayed with corresponding p-value.
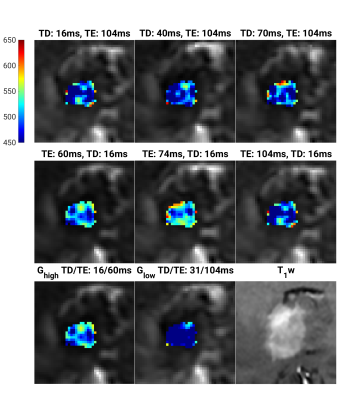
Fig. 3 ADC parameters maps, for b = 100 and 1000 s/mm2 from a patient with a diffuse large B-cell lymphoma. Maps for the three different diffusion times (first row), three different echo time (second row), and two gradient strengths (third row). A post contrast T1-weighted image of the tumor is shown in the bottom right corner. While no visible change in ADC with diffusion time (first row) are present, ADC decreases with increasing TE (middle row) and decreases with decreasing gradient strength (third row). For TD/T E =16/70ms the mean ADCb100,1000 was 505 ± 35 x10-6 mm2/s.
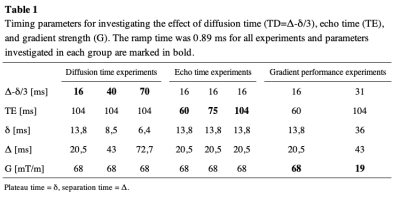
Table 1
DOI: https://doi.org/10.58530/2023/1750