1747
Classification of IDH mutation with Arterial Spin Labeling and Dynamic Susceptibility Contrast MRI in adult gliomas1Department of Pathophysiology, the Second Faculty of Medicine, Charles University, Prague, Czech Republic, 2Laboratory of Developmental Epileptology, Institute of Physiology, Czech Academy of Sciences, Prague, Czech Republic, 3Department of Physics and Computational Radiology, Division of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 4Oslo University Hospital, Department of Neurosurgery, Oslo, Norway, 5Department of Circuit Theory, Faculty of Electrical Engineering, Czech Technical University, Prague, Czech Republic, 6Department of Neurosurgery, Amsterdam University Medical Center, Amsterdam, Netherlands, 7Instituto Universitario de Tecnologías de la Información y Comunicaciones, Universitat Politècnica de València, Valencia, Spain, 8Department of Oncology, Oslo University Hospital, Oslo, Norway, 9Institute for Cancer Genetics and Informatics, Oslo University Hospital, Oslo, Norway, 10Institute of Radiopharmaceutical Cancer Research, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 11Department of Radiology and Nuclear Medicine, Cancer Center Amsterdam, Amsterdam University Medical Center, Amsterdam, Netherlands
Synopsis
Keywords: Tumors, Perfusion, Genetics
IDH genotype status is an important marker in glioma diagnostics. Given that IDH mutation affects the tumor vascularization pattern, perfusion imaging has the potential to become a non-invasive tumor histopathology assessor. In this study, two methods of perfusion MRI – Dynamic Susceptibility Contrast (DSC) and Arterial Spin Labeling (ASL) – were compared in their ability to assess IDH mutation status. DSC- and ASL-derived perfusion maps correlated significantly and were feasible parameters in the IDH classification task. Mean tumor CBF quantified with ASL had the highest AUC score, sensitivity, and specificity, supporting the feasibility of using ASL in clinical glioma diagnostics.INTRODUCTION
Glioma is the most common malignant primary brain tumor. The latest 2021 World Health Organization (WHO) classification of CNS tumors emphasizes the importance of molecular markers for tumor classification [1], especially isocitrate dehydrogenase (IDH) mutation status. IDH mutation is characterized by a different tumor vascularization pattern compared to IDH-wildtype [2] and is associated with a better prognosis. The higher vascularization in IDH-wildtype gliomas is represented by an increased cerebral blood flow (CBF), a potential non-invasive surrogate marker of IDH-genotype classification, as previously shown with Dynamic Susceptibility Contrast (DSC) [3] and Arterial Spin Labeling (ASL) MRI [4]. While pilot results of both methods showed the feasibility of assessing the IDH mutation status, their performance was never directly compared. Given concerns about the safety of gadolinium-based contrast agents and higher costs and invasiveness of DSC, there is a strong interest in validating ASL as an alternative to DSC in clinical diagnostics of glioma. This study aims to evaluate and directly compare the ability of DSC and ASL perfusion MRI to assess IDH mutation status.METHODS
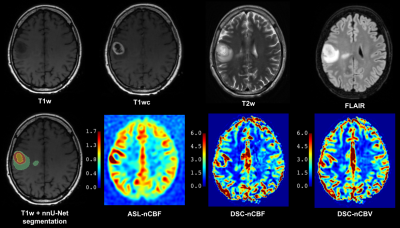
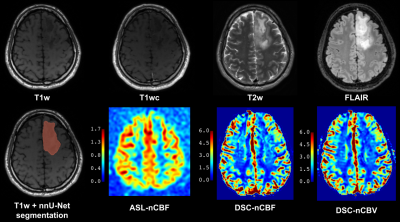
Seventy-three patients (53.4±16.2years, 48 men) admitted to Oslo University hospital between 2016-2021 were retrospectively included. Inclusion criteria were: age≥18y, histologically confirmed glioma, ASL and DSC scans acquired within one session, and no prior cancer treatment. Data were acquired on 3T GE MRI scanners. The protocol included T1-weighted 3D FSPGR/BRAVO before (T1w) and after contrast-agent application (T1wc), T2w 3D FLAIR (FSE/CUBE), T2w 2D Propeller, background-suppressed 3D FSE spiral pCASL (labeling duration 1450 ms, post-labeling delay 2025 ms, TR/TE 5025/11.072 ms, 4×4×3.0 mm3 voxels, and a separate M0 scan) and one-bolus 2D GRE-EPI DSC (baseline duration 7 s, total duration 84 s, TR/TE 1875/45 ms, flip angle 60°, 1.875×1.875×6.0 mm3 voxels). IDH-mutation and 1p/19q co-deletion status were collected from the clinical database when available. Tumor and edema were segmented with the nnU-Net segmentation model [5] from T1wc, T1w, T2w, and FLAIR. ASL images were processed with ExploreASL version 1.9.0 [6], including healthy tissue segmentation excluding tumor and necrotic areas, motion correction, and quantification [7]. ASL-CBF maps were normalized to mean CBF in normal GM (nCBF). DSC scans were processed in nordicICE (v4.1.2; NordicNeuroLab) with the use of whole-volume arterial input function, motion and leakage correction, and normalization to automatically-detected normal-appearing WM (nCBF/nCBV) [8]. Subjects without structural scans (n=2), poor ASL quality (n=5), failed normal tissue segmentation (n=5), failed tumor segmentation (n=3), or missing IDH-mutation status (n=13) were excluded. Mean and maximum (95th percentile) perfusion values were extracted from the tumor region of interest (ROI) and statistically analyzed with a t-test, receiver operating characteristic (ROC) curve, and Pearson’s correlation analysis in MATLAB.RESULTS
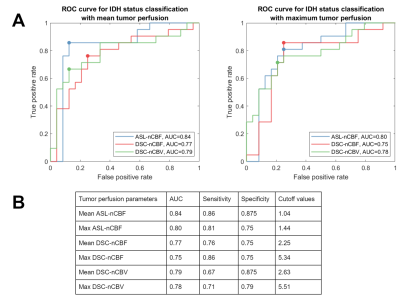
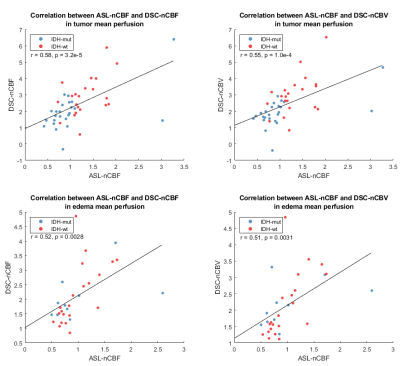
The final dataset included 45 subjects (52.2±16.7years, 30 men), out of which 24/21 were IDH-mutant/wildtype, 31 had cerebral edema, and 11/9 had negative/postive 1p/19q co-deletion status.Table 1 summarizes the perfusion parameters in IDH-mutant and IDH-wildtype gliomas. IDH-wildtype gliomas have significantly higher maximum tumor ASL-nCBF, DSC-nCBF, DSC-nCBV and mean tumor DSC-nCBF, DSC-nCBV.Figure 1 shows ROC curves of ASL and DSC perfusion parameters for IDH genotype classification. Mean tumor ASL-nCBF shows the best performance with the highest area under the curve (AUC), sensitivity, and specificity. ROI-wise linear correlation analysis visualized in Figure 2 shows a significant positive correlation between ASL and DSC perfusion parameters in both tumor and edema.DISCUSSION
Mean tumor ASL-nCBF did not significantly differ between IDH-wildtype and IDH-mutant gliomas, but it showed the best performance in IDH classification. The possible explanation for this inconsistency is the presence of outlying mean tumor hyperperfusion in the dataset. AUC scores in this study are comparable with the reported values by Wang et al. (0.74/0.8 for mean/maximum tumor rCBF) [4]. The sensitivity and specificity values are also comparable to those reported by Wang et al. (sensitivity/specificity/cutoff of mean and maximum tumor rCBF 0.867/0.703/0.89 and 0.865/0.733/1.25) and by Brendle et. al [9] (sensitivity/specificity/cutoff of native ASL-CBF in astrocytoma 0.75/0.88/9.2), but ASL-nCBF cutoff thresholds differ. Possible explanations for it are manual segmentations of ROI, different ASL-processing software, and datasets limited by specific tumor subtypes in previous studies. Performance of DSC-derived markers was inferior to ASL, but still in concordance with previous studies (pooled AUC score 0.82 with 95%-confidence interval: 0.66–0.93) [3]. Significant positive correlation between ASL and DSC markers in tumor is in concordance to the results reported by Ma et al. [10] (correlation between ASL-rCBF and DSC-rCBF r=0.580, p<0.01). We have additionally investigated correlation of ASL and DSC in edema to evaluate the potential of ASL for assessing tumor microenvironment heterogeneity [11]. The results of correlation analysis show promise for ASL in assessing tumor habitats.There are several limitations. The use of advanced statistical methods is recommended for improved IDH genotype classification. Advanced assessment of tumor perfusion heterogeneity has a potential of increasing classification performance [11]. The accuracy of CBF estimation can be limited with arterial transit time artifacts, which might be prominent in single-delay ASL. Additionally, normalization of CBF maps can be biased with improper definition of healthy tissue done by automatic pipelines.
CONCLUSION
Mean tumor ASL-nCBF shows better performance in IDH-genotype classification than DSC. ASL and DSC show significant positive correlation that underlines the feasibility of ASL in clinical diagnostics.Acknowledgements
This publication is part of the COST Action CA18206 Glioma MR Imaging 2.0, supported by COST (European Cooperation in Science and Technology) www.cost.eu. It is supported by project nr. LX22NPO5107 (MEYS): Financed by EU – Next Generation EU and by grant nr. NU21-08-00228 from Czech Health Research Council.References
1. WHO Classification of Tumours Editorial Board. World Health Organization Classification of Tumours of the Central Nervous System. 5th ed.Lyon: International Agency for Research on Cancer; 2021.
2. Álvarez-Torres, MdM, Lopez-Cerdan, A., de la Iglesia Vayá, V., Fuster-Garcia, E., Garcia-Garcia, F., & Garcia-Gomez, J. M. (2022). Vascular differences between glioblastoma IDH-wildtype and astrocytoma IDH-mutant grade 4 at imaging and transcriptomic level.
3. van Santwijk, L., Kouwenberg, V., Meijer, F., Smits, M., & Henssen, D. (2022). A systematic review and meta-analysis on the differentiation of glioma grade and mutational status by use of perfusion-based magnetic resonance imaging. Insights into Imaging, 13(1), 1-12.
4. Wang, N., Xie, S. Y., Liu, H. M., Chen, G. Q., & Zhang, W. D. (2019). Arterial spin labeling for glioma grade discrimination: correlations with IDH1 genotype and 1p/19q status. Translational Oncology, 12(5), 749-756.
5. Isensee, F.; Petersen, J.; Klein, A.; Zimmerer, D.; Jaeger, P.F.; Kohl, S.; Wasserthal, J.; Koehler, G.; Norajitra, T.; Wirkert, S.; et al. nnU-Net: Self-adapting framework for u-net-based medical image segmentation. arXiv 2018, arXiv:1809.10486
6. Mutsaerts, H. J., Petr, J., Groot, P., Vandemaele, P., Ingala, S., Robertson, A. D., ... & Barkhof, F. (2020). ExploreASL: an image processing pipeline for multi-center ASL perfusion MRI studies. Neuroimage, 219, 117031.
7. Alsop, D. C., Detre, J. A., Golay, X., Günther, M., Hendrikse, J., Hernandez‐Garcia, L., ... & Zaharchuk, G. (2015). Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine, 73(1), 102-116.
8. Emblem, K. E., & Bjornerud, A. (2009). An automatic procedure for normalization of cerebral blood volume maps in dynamic susceptibility contrast− based glioma imaging. American journal of neuroradiology, 30(10), 1929-1932.
9. Brendle, C., Hempel, J. M., Schittenhelm, J., Skardelly, M., Tabatabai, G., Bender, B., ... & Klose, U. (2018). Glioma grading and determination of IDH mutation status and ATRX loss by DCE and ASL perfusion. Clinical Neuroradiology, 28(3), 421-428.
10. Ma, H., Wang, Z., Xu, K., Shao, Z., Yang, C., Xu, P., ... & Rong, Y. (2017). Three-dimensional arterial spin labeling imaging and dynamic susceptibility contrast perfusion-weighted imaging value in diagnosing glioma grade prior to surgery. Experimental and therapeutic medicine, 13(6), 2691-2698.
11. Juan-Albarracín, J., Fuster-Garcia, E., García-Ferrando, G. A., & García-Gómez, J. M. (2019). ONCOhabitats: A system for glioblastoma heterogeneity assessment through MRI. International journal of medical informatics, 128, 53-61.
Figures