1745
Tumor habitat-derived diffusion spectrum imaging features in evaluating gliomas-A comparative study with DKI1Department of Radiology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, China, 2Department of Radiology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China, 3MR Scientific Marketing, Siemens Healthineers Ltd., Guangzhou, Guangdong, China
Synopsis
Keywords: Tumors, Tumor
Currently, in diffuse glioma, in addition to histopathology, the molecular features have an important role in glioma diagnosis. As a new computing framework for diffusion spectrum imaging (DSI), mean apparent propagator (MAP) could investigate more subtle information of tumor microstructures than the Gaussian diffusion models, while MAP has not been studied much in glioma. Besides, the analysis of tumor habitat could reflect the glioma heterogeneity. Hence, we aim to analyze the tumor habitat-derived MAP metrics and further explore their clinical value in glioma evaluation, meanwhile, we introduce Diffusion-kurtosis imaging (DKI) as a reference to compare the diagnostic efficiency of DSI.Introduction/Purpose
Diffuse glioma is the most frequent malignant primary brain tumor, with an invariably fatal clinical course. Currently, in addition to histopathology, the molecular features have an important role in glioma diagnosis, such as the isocitrate dehydrogenase (IDH) genotype and 1p/19q codeletion status [1]. Finding useful noninvasive imaging methods to preoperatively provide glioma pathology information has important clinical value. Diffusion MRI could provide pathology information by evaluating glioma microstructure at a relatively high spatial resolution [2-5]. Diffusion-kurtosis imaging (DKI) was able to describe the non-Gaussian distribution of water molecules, and showed the higher diagnostic efficacy in grading glioma and identifying IDH-1 mutation status [6-8]. Meanwhile, as a new computing framework for diffusion spectrum imaging (DSI), mean apparent propagator (MAP) MRI could investigate subtle information of tumor microstructures [9]. Quantitative MAP parameters have been reported could enabled differentiation between high-grade gliomas and solitary brain metastases, evaluate gliomas grades and cellular proliferation, as well as IDH mutation and 1p/19q genotyping [10-12]. Gao A illustrated that MAP provide more significant tumor microstructure information than diffusion tensor imaging (DTI) and DKI [5]. So far, the sample size of the studies about the MAP metrics in glioma diagnosis was generally small [10; 12]. Besides, those reports rarely refered to glioma genotyping using MAP metrics. Although IDH mutation prediction has been mentioned, another critical molecular markers identification, the 1p/19q genotypes, was commonly lack [10; 11]. Conclusively, bigger sample size and more analytical method need to consider to further verify and supplement existing findings. Meanwhile, the analysis of tumor habitat in diffuse glioma, which included tumour parenchyma, peritumoral edema and tumor necrosis regions, could reflect the lesion heterogeneity [13-15]. We aim to analyze the tumor habitat-derived diffusion spectrum imaging features (MAP-MRI metrics), further, to explore the clinical value of these features in glioma evaluation. Additionally, we introduce DKI as a reference to compare the diagnostic efficiency of DSI.Methods
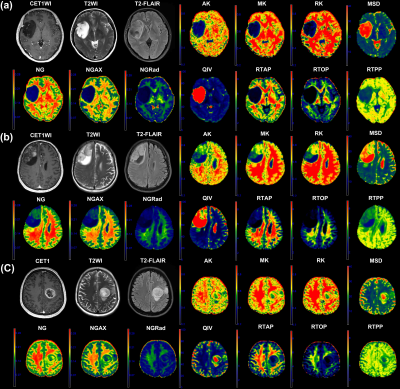
Seventy glioma patients underwent conventional and diffusion MRI by 3T MRI system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany, a 64-channel head coil) were enrolled. By using NeuDiLab software developed in-house based on the openresource tool DIPY (Diffusion Imaging in Python, http:// nipy.org/dipy), the MAP and DKI metrics derived from tumor habitats, including tumour parenchyma (TP), peritumoural areas (PT), and contralateral normal-appearing white matter regions, were obtained and analyzed. Those metric values were correlated with tumor grades, IDH mutation and 1p/19q codeletion status, meanwhile, the diagnostic performance was assessed and compared between DKI and MAP. Besides, random forest (RF) models were constructed to further assess the diagnostic efficiency.Results
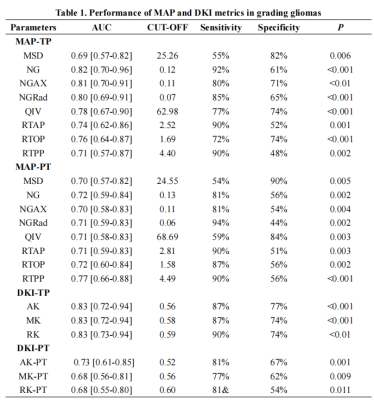
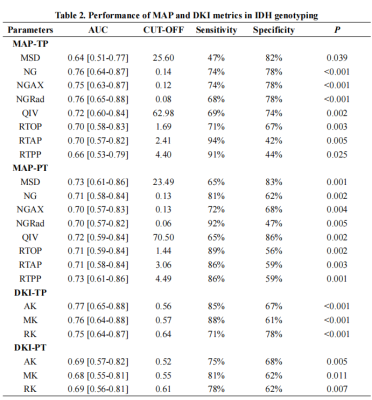
In TP areas, MSD and QIV were significantly lower, whereas NG, NGAX, NGRad, RTAP, RTOP, RTPP, MK, RK and AK were higher in high-grade gliomas than those in low-grade gliomas, as well as in IDH mutation gliomas than IDH wildtype gliomas, while in PT areas, those trends was totally reversed. For tumor grading, in the TP area, MK, AK and RK performed best (AUC, 0.83), in the PT areas, RTPP performed best (AUC, 0.77). For IDH genotyping, in the TP area, AK performed best (AUC, 0.77), in the PT area, MSD and RTPP performed best (AUC, 0.73). RF models proven DKI was more important in TP areas whereas MAP was more important in the PT areas. Only RTPP and NGRad in the PT could distinguish grade 3 and 4 gliomas (AUC, 0.75). RK in TP areas performed best in differenting oligodendroglioma of LGGs from other gliomas (AUC, 0.74). No significant differences of AUCs between DKI and MAP.Discussion/Conclusion
Our results demonstrated that all the DKI and MAP metrics in tumor habitats showed significant value in glioma grading and and IDH genotyping. Comparing to previous researches, our study demonstrated more significant MAP metrics, such as RTPP [16] and NG [10], besides, our study supplemented the corresponding results for the PT areas, however, most previous studies mainly focused on TP areas [2; 3; 10; 16]. Additionally, in TP areas, mainly the DKI metrics showed the highest diagnostic effectivity, while in PT areas, mainly the MAP metrics, especially RTPP, showed the highest diagnostic effectivity, and our random forest models further proven that the DKI was more important in TP areas whereas MAP was more important in the PT areas, these results reminding us that, comparing to DKI, MAP may be more sensitive to the microstructural changes in PT areas, while DKI metrics were more sensitive than MAP to the microstructural changes in TP areas. In our study here, not only the DKI in TP areas but also the QIV in TP areas were proven to be capable of distinguishing oligodendroglioma from astrocytoma. Our results did not find a difference of efficiency between DKI and MAP in glioma diagnosis.In conclusion, tumor habitat-derived DSI features can effectively evaluate gliomas, while comparing to DKI, there was no significant differences. In different tumor habitats, the priority of diagnostic value of DKI and MAP metrics was different. MAP, especially RTPP, might be a promising indicator in exploring glioma PT areas.
Acknowledgements
This study was supported by the Guangdong Basic and Applied Basic Research Foundation, China (No.2020A1515011436, 2021A1515012279, 2022A1515011264) and the National Natural Science Foundation (NSFC 82172015).References
1. Gritsch S, Batchelor TT, Gonzalez CL (2022) Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 128:47-58
2. Qi XX, Shi DF, Ren SX et al (2018) Histogram analysis of diffusion kurtosis imaging derived maps may distinguish between low and high grade gliomas before surgery. European Radiology 28:1748-1755
3. Chu JP, Song YK, Tian YS et al (2021) Diffusion kurtosis imaging in evaluating gliomas: different region of interest selection methods on time efficiency, measurement repeatability, and diagnostic ability. European Radiology 31:729-739
4. Wang P, Weng L, Xie S et al (2021) Primary application of mean apparent propagator-MRI diffusion model in the grading of diffuse glioma. European Journal of Radiology 138:109622
5. Gao A, Zhang H, Yan X et al (2022) Whole-Tumor Histogram Analysis of Multiple Diffusion Metrics for Glioma Genotyping. Radiology 302:652-661
6. Xu Z, Ke C, Liu J et al (2021) Diagnostic performance between MR amide proton transfer (APT) and diffusion kurtosis imaging (DKI) in glioma grading and IDH mutation status prediction at 3T. European Journal of Radiology 134:109466
7. Van Cauter S, Veraart J, Sijbers J et al (2012) Gliomas: diffusion kurtosis MR imaging in grading. Radiology 263:492-501
8. Zhao J, Wang YL, Li XB et al (2019) Comparative analysis of the diffusion kurtosis imaging and diffusion tensor imaging in grading gliomas, predicting tumour cell proliferation and IDH-1 gene mutation status. J Neurooncol 141:195-203
9. Le H, Zeng W, Zhang H et al (2020) Mean Apparent Propagator MRI Is Better Than Conventional Diffusion Tensor Imaging for the Evaluation of Parkinson's Disease: A Prospective Pilot Study. Frontiers in Aging Neuroscience 12:563595
10. Sun Y, Su C, Deng K, Hu X, Xue Y, Jiang R (2022) Mean apparent propagator-MRI in evaluation of glioma grade, cellular proliferation, and IDH-1 gene mutation status. European Radiology 32:3744-3754
11. Guo H, Liu J, Hu J et al (2022) Diagnostic performance of gliomas grading and IDH status decoding A comparison between 3D amide proton transfer APT and four diffusion-weighted MRI models. Journal of Magnetic Resonance Imaging
12. Mao J, Zeng W, Zhang Q et al (2020) Differentiation between high-grade gliomas and solitary brain metastases: a comparison of five diffusion-weighted MRI models. Bmc Medical Imaging 20:124
13. Beig N, Bera K, Prasanna P et al (2020) Radiogenomic-Based Survival Risk Stratification of Tumor Habitat on Gd-T1w MRI Is Associated with Biological Processes in Glioblastoma. Clinical Cancer Research 26:1866-1876
14. Verma R, Correa R, Hill VB et al (2020) Tumor Habitat-derived Radiomic Features at Pretreatment MRI That Are Prognostic for Progression-free Survival in Glioblastoma Are Associated with Key Morphologic Attributes at Histopathologic Examination: A Feasibility Study. Radiol Artif Intell 2:e190168
15.Prasanna P, Patel J, Partovi S, Madabhushi A, Tiwari P (2017) Radiomic features from the peritumoral brain parenchyma on treatment-naive multi-parametric MR imaging predict long versus short-term survival in glioblastoma multiforme: Preliminary findings. European Radiology 27:4188-4197
16. Xie SH, Lang R, Li B et al (2022) Evaluation of diffuse glioma grade and proliferation activity by different diffusion-weighted-imaging models including diffusion kurtosis imaging (DKI) and mean apparent propagator (MAP) MRI. Neuroradiology
Figures