1743
Combination of DKI and IVIM for differentiating True Progression from Pseudoprogression of Glioma with Postoperative Radiation Therapy
Pei Dang1, Xueying Huang2, Zhihua Yang3, Aijun Wang2, Lidong Wang4, Yuhui Xiong5, Xuhong Yang6, Minglei Wang2, and Xiaodong Wang7
1Radiology, General Hospital of Ningxia Medical University, yinchuan, China, 2General Hospital of Ningxia Medical University, Yinchuan, China, 3Radiotherapy, General Hospital of Ningxia Medical University, Yinchuan, China, 4Radiology, Yinchuan Hospital of Traditional Chinese Medicine, Yinchuan, China, 5GE Healthcare MR Research, Beijing, China, 6Ningxia Medical University, Yinchuan, China, 7Radiology, General Hospital of Ningxia Medical University, Yinchuan, China
1Radiology, General Hospital of Ningxia Medical University, yinchuan, China, 2General Hospital of Ningxia Medical University, Yinchuan, China, 3Radiotherapy, General Hospital of Ningxia Medical University, Yinchuan, China, 4Radiology, Yinchuan Hospital of Traditional Chinese Medicine, Yinchuan, China, 5GE Healthcare MR Research, Beijing, China, 6Ningxia Medical University, Yinchuan, China, 7Radiology, General Hospital of Ningxia Medical University, Yinchuan, China
Synopsis
Keywords: Tumors, Cancer, Glioma;True Progression;Pseudoprogression;DKI;IVIM
The differentiation of pseudoprogression from true progression remains a crucial diagnostic dilemma in glioma with postoperative radiation therapy.This work to explore the role of DKI combined IVIM in differentiating true progression and pseudoprogression in glioma. It was concluded that the MK and RK from DKI、D and D* from IVIM can be used as novel imaging biomarkers for Clinical and radiologists in differentiating glioma true progression from pseudoprogression. Combining MK, RK, D and D* may explore as an effective strategy to improve the ability for discriminating glioma true progression and pseudoprogression.Introduction
Glioma is the most common malignant brain tumor in adult and is extremely aggressive. To date, present standard therapy includes surgical approaches, such as gross total or subtotal excision,followed by concomitant chemo-radiotherapy and temozolomide adjuvant chemotherapy1.However, such treatment may cause radiation-induced damage to brain tissue of glioma patients and increase the risk of recurrence. Pseudoprogression is a sub-acute clinical entity, which is characterized by the expansion of existing lesions or the appearance of new lesions within 12 weeks after radiation therapy2. Given its ability to mimic true tumour development, PsP can lead to interference with treatment course or unwarranted surgical intervention. Thus, its prompt and definitive detection serves a critical role in glioma management3.At present, the diagnosis of gliomas true progression mainly depends on histopathology, and the tumor tissue needs to be obtained by biopsy or surgical excision, which is not only invasive, but also subject to sampling bias and it may not be representative of the intra-tumoral heterogeneity.Many studies have used various advanced imaging techniques to evaluate treatment-related responses,Several studies had evaluate the treatment response in an early stage by using noninvasive imaging,such as diffusion-weighted imaging 4,5and dynamic susceptibility-weighted contrast en-hancement measures6,7.Diffusion kurtosis imaging (DKI) may reflect tissue complexity by using higher b values. By using multiple b values, intravoxel incoherent motion (IVIM) may help evaluate tissue blood perfusion and provide more accurate characterization of diffusion motion. Thus, the purpose of this study was to investigate the value of DKI and IVIM in differentiating true progression and pseudoprogression in glioma.Materials and Methods
Patients:The prospective study was approved by the institutional ethics committee. Written informed consent was obtained from all patients.A total 40 patients ranging from 21 to 65 years old (49.6±11.2 years) were enrolled between August 2018 and September 2021.Figure1 illustrates the detailed patient enrollment process.Imaging parameters:All MRI examinations were performed using the PHILIPS Medical Systems/Ingenia 3.0 T MRI scanner,with a 48-channel phased-array head coi.(1)DKI: TR/TE = 3809/98ms, FOV = 230×230mm, matrix =84×84, slice thickness = 6 mm; acquisition time = 6min and 30s;(2)IVIM:TR/TE = 2727/98ms, FOV = 230×230mm, matrix =128×128, slice thickness =6 mm; acquisition time = 3min and 20 s, details for Table 1 .
Data processing:DKI sequence:Import DKI data into Diffiisional Kurttosis Estimator(DKE, https://www.nitrc.org/projects/dke).Diagram of DKI parameters MK, RK, AK, MD, FA,are shown in Fig.2 .IVIM sequence:Import IVIM data into FireVoxel (https://firevoxel.org/download/).Diagram of IVIM parameters ADC,D,D*,f are shown in Fig.2. Use ITK-SNAP(http://www.itksnap.org/pmwiki/pmwiki.php) sketch ROI.The region of interest is performed at the largest level of the enhancement lesion (T1 axial enhancement sequence).ROI delineation and measurement avoids necrosis, cystization, bleeding and proximity of normal tissue. The ROI range is determined by the size of the tumor parenchyma and is repeated The average is taken 3 times, and the data is kept to two decimal places.
Statistical analysis:The Student’s t-test, Mann-Whitney U-test or Fisher’s exact test was used to compare the parameters between true progression and pseudoprogression. Receiver operating characteristic (ROC) curves (AUC) were also evaluated to assess the diagnostic value of parameters for discrimination.A p-value0.05 was considered significant.
Results
1.The MK,RK and D* values were significantly higher in the enhancing lesions of glioma true progression compared to pseudoprogression (P < 0.001,P < 0.001),and the D values for glioma recurrence were significantly lower than those of PsP (P = 0.006). The AK, MD and FA values in the enhancing lesions did not differ significantly between groups (P > 0.05) (Table 2).2.The DKI and IVIM parametric maps of glioma between groups are shown in Figure 2.
3.Combining MK, RK, D and D* showed highest sensitivity (0.958) and specificity (0.875),show in Figure 3.
Discussion
DKI can reflect the most real non-Gaussian movement mode of water molecules in the tissues 8. The MK and RK in the enhancing lesion center are mainly determined by tumor cell density and vascularity.At pathology, high-grade glioma true progression may be characterized by proliferation of tumor cells, increased cellularity, and vascular hyperplasia, while PsP may be related to tissue inflammation, vascular dilation, disorders of the blood-brain barrier, and vasogenic edema9. MK、RK in true progression were significantly higher than those in pseudoprogression, Therefore, we hypothesized that MK and RK values in enhancing lesions would accurately reflect the microstructural differences between glioma true progression and pseudoprogression.IVIM allows the simultaneous acquisition of diffusion and perfusion parameters which reflecttumor cellularity and vascularity,respectively10.D is the diffusion parameter which reflects the diffusion coefficient for water while D* represents perfusion-related diffusion. True progression is characterized by vascular proliferation, manifested by elevated tumor vasculature density,this phenomenon is reflected in the change in D*.In addition, the MD value in true progression group was significantly lesser than that in the pseudoprogression group. This showed that the D may more accurately reflect the limited diffusion movement of water molecules caused by complex components in the tissue.Conclusion
The results of this study may demonstrate the potential value of using multimodal MRI techniques to differentiate true progression from pseudoprogression in its early stages to help decision making in early intervention and improve the prognosis of glioma.Acknowledgements
Thanks to the Department of Radiology, General Hospital of Ningxia Medical University for the equipment provided and Professor Wang Xiaodong for the guidance.References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
- Lieberman F. Glioblastoma update: molecular biology, diagnosis, treatment, response assessment, and translational clinical trials. F1000Res. 2017;6:1892. Published 2017 Oct 26.
- Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963-1972.
- Chu HH, Choi SH, Ryoo I, et al. Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology. 2013;269(3):831-840.
- Qian X, Tan H, Zhang J, Zhao W, Chan MD, Zhou X. Stratification of pseudoprogression and true progression of glioblastoma multiform based on longitudinal diffusion tensor imaging without segmentation. Med Phys. 2016;43(11):5889.
- Suh CH, Kim HS, Choi YJ, Kim N, Kim SJ. Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. AJNR Am J Neuroradiol. 2013;34(12):2278-2286.
- Yun TJ, Park CK, Kim TM, et al. Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging. Radiology. 2015;274(3):830-840.
- Nogueira L, Brandão S, Matos E, et al. Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol. 2014;24(6):1197-1203.
- Strauss SB, Meng A, Ebani EJ, Chiang GC. Imaging Glioblastoma Posttreatment: Progression, Pseudoprogression, Pseudoresponse, Radiation Necrosis. Radiol Clin North Am. 2019;57(6):1199-1216.
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497-505.
Figures
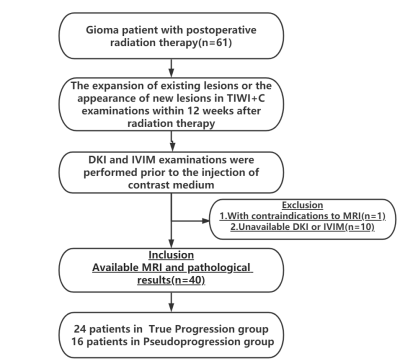
Fig 1.Schematic illustration of the patient-selection criteria.
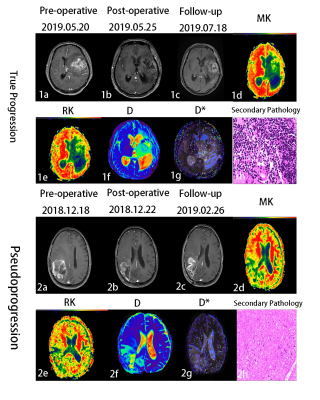
Fig 2.1a-1h:43-year-old glioma patient with true progression.T1WI+C (1a-1c), 1c shows an abnormal enhancement lesion in the left temporal lobe,the corresponding part of the enhancement lesion in MK map1d,RK map1e, D map1f and D*map 1g.1h (HE×200) shows the diffuse distribution of cancer cells. 2a-2h:56-year-old glioma patient withpseudoprogression.T1WI+C(2a-2c), 2c shows an abnormal enhancement lesion in the right frontal lobe,the corresponding part of the enhancement lesion in MK map 2d,RKmap 2e,D map 2f and D*map 2g.2h (HE×200) shows no tumor cells.
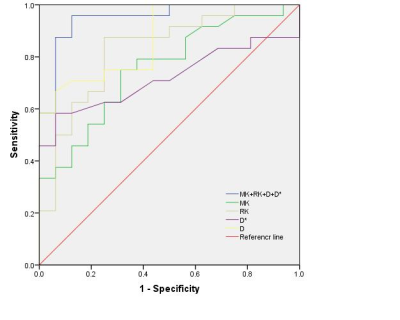
Fig 3.The ROC of MK, RK, D*,D and MK+RK+D + D* values for differentiation of glioma true progression and pseudoprogression.The AUC of MK+RK+D + D* (0.951) is significantly higher than MK (0.755) , RK(0.828),D*(0.720)and D (0.923) (P = 0.003, 0.001, 0.001,0.002 respectively).
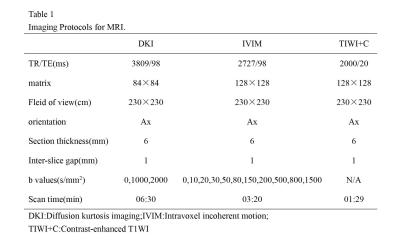
Table 1 Imaging Protocols for MRI.
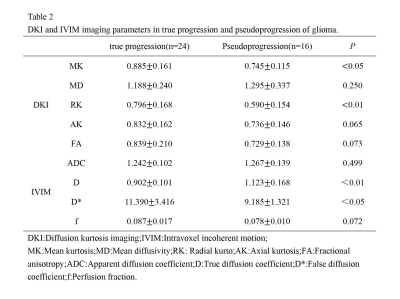
Table 2 DKI and IVIM imaging parameters in true progression and pseudoprogression of glioma.
DOI: https://doi.org/10.58530/2023/1743