1741
Time-dependent diffusion-weighted imaging for differentiation between glioblastoma and brain metastasis
Kiyohisa Kamimura1, Tsubasa Nakano1, Tomohito Hasegawa1, Masanori Nakajo1, Hiroyuki Uchida2, Takashi Iwanaga3, Hiroshi Imai4, Thorsten Feiweier5, and Takashi Yoshiura1
1Radiology, Kagoshima University, Kagoshima, Japan, 2Neurosurgery, Kagoshima University, Kagoshima, Japan, 3Radiological Technology, Kagoshima University Hospital, Kagoshima, Japan, 4Siemens Healthcare K.K., Tokyo, Japan, 5Siemens Healthcare GmbH, Erlangen, Germany
1Radiology, Kagoshima University, Kagoshima, Japan, 2Neurosurgery, Kagoshima University, Kagoshima, Japan, 3Radiological Technology, Kagoshima University Hospital, Kagoshima, Japan, 4Siemens Healthcare K.K., Tokyo, Japan, 5Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Keywords: Tumors, Diffusion/other diffusion imaging techniques
To investigate the utility of time-dependent DWI for differentiating between glioblastoma and brain metastasis, 65 patients with glioblastoma and 27 patients with brain metastasis were examined. ADC was not significantly different between the two tumor types neither at a short (7.1ms) nor at a long (44.5ms) diffusion time, whereas the ADC difference (ΔADCmean, ΔADC5, ΔADC95) and ADC change ratio (rADCmean, rADC5, rADC95) were significantly higher in brain metastasis than in glioblastoma. The ΔADCmean showed the best diagnostic performance. The ΔADCmean and rADCmean showed a significant negative correlation with ADC44.5msmean, but not with extracellular extravascular space volume fraction derived from DCE-MRI.INTRODUCTION
Preoperative differentiation between brain metastasis and glioblastoma is clinically important, but is often problematic as they can show similar characteristics on conventional MR images. There have been controversies regarding the abilities of conventional ADC values to differentiate brain metastases from glioblastomas.1,2 Complementary to conventional pulsed gradient spin-echo sequences (PGSE), oscillating gradient spin-echo (OGSE) sequences3 allow for DWI with a short diffusion time, enabling time-dependent diffusion analysis which could provide specific information regarding restricted diffusion. Our purposes were to investigate the utility of time-dependent DWI parameters in distinguishing brain metastases from glioblastomas, and to examine their correlation with extravascular extracellular space (ve).METHODS
A retrospective study was performed including 27 patients with brain metastasis (mean age, 68 ± 10 years) and 65 patients with glioblastoma (69 ± 13 years). All patients underwent preoperative MR imaging using a 3T system (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany) with a 20-channel head/neck coil. Time-dependent DW images were acquired using research DWI sequences with OGSE1 using sine-modulated trapezoidal waveforms (effective diffusion time = 7.1 ms) and PGSE (44.5 ms) with b values of 0 and 1500 s/mm2. Maps of the difference in ADC values between OGSE and PGSE sequences were generated: ΔADC = ADC7.1ms - ADC44.5ms. In addition, maps of the ratio of ADC change between OGSE and PGSE sequences were generated: rADC = (ADC7.1ms - ADC44.5ms)/ADC44.5ms × 100 (%). Moreover, maps of the volume of extravascular extracellular space per unit volume of tissue (ve) were obtained using DCE-MRI. ROI analysis was performed by two independent radiologists to measure the ADCs at the two diffusion times (ADC7.1ms and ADC44.5ms), ΔADC, rADC, and ve (Fig. 1). The interobserver agreement regarding parametric measurements by the two observers was analyzed by calculating the intraclass correlation coefficient (ICC). Measurements by the two observers for each patient were averaged for further analysis. The mean, fifth percentile, and 95th percentile values of each parameter were compared between brain metastases and glioblastomas using the Mann-Whitney U test. In addition, the diagnostic performances of the parameters were evaluated using ROC curve analysis. Furthermore, we examined the correlation of the time-dependent parameters with ADCs and ve using Pearson’s correlation coefficient.RESULTS
The agreement of the two observers was excellent for all measures (ICCs ranged from 0.825 to 0.981). The results of the parametric comparisons are shown in Figure 2. No significant difference was noted between glioblastomas and brain metastases in any of the three indices of ADC44.5ms and ADC7.1ms. ΔADCmean (P<0.01), ΔADC5 (P<0.05), ΔADC95 (P<0.01), rADCmean (P<0.01), rADC5 (P<0.01), and rADC95 (P<0.01) were significantly higher in brain metastases than in glioblastomas, whereas the ve5 (P<0.05) values were significantly lower for brain metastases than for glioblastomas. The ROC curve analysis showed significance for ΔADCmean (AUC=0.877, P<0.01), ΔADC95 (0.865, <0.01), rADCmean (0.819, <0.01), rADC5 (0.652, 0.02), rADC95 (0.796, <0.01), and ve5 values (0.630, 0.04). Considering ADC44.5ms, ADC7.1ms, ΔADC, rADC, and ve values, the highest AUC was obtained for ADC44.5ms5, ADC7.1ms95, ΔADCmean, rADCmean, and ve5. Pairwise comparisons revealed that AUCs of ΔADCmean and rADCmean were significantly greater than that of ADC44.5ms5 (each P<0.001). The ROC curves for the ADC44.5ms5, ADC7.1ms95, ΔADCmean, and rADCmean are shown in Figure 3. The ΔADCmean (r=-0.309, P<0.01) and rADC mean (-0.618, <0.01) showed a significant negative correlation with ADC44.5msmean, but not with ve5 (r=-0.071, -0.006, P=0.50, 0.95).DISCUSSION
Our results suggest that time-dependent DWI parameters, especially ΔADCmean, are useful imaging markers for distinguishing brain metastases from glioblastomas, while ADC itself is not. As the majority of brain metastases are composed of epithelial cells, the cell gaps are very narrow due to the cell junction system, while glioblastomas are composed of heterogeneous cells with extracellular matrix and fine hemorrhage and necrosis. Theoretically, narrower extracellular space could explain stronger diffusion time-dependence of ADC in brain metastasis. However, we did not find significant correlation between the time-dependent DWI parameters and ve derived from DCE-MRI. Further studies are needed to elucidate the pathological factors that account for our findings.CONCLUSION
The time-dependent DWI parameters, especially ΔADCmean may be useful in distinguishing brain metastases from glioblastomas.Acknowledgements
No acknowledgement found.References
1. Cindil E, Sendur HN, Cerit MN, et al. Validation of combined use of DWI and percentage signal recovery-optimized protocol of DSC-MRI in differentiation of high-grade glioma, metastasis, and lymphoma. Neuroradiology. 2021;63:331–342.
2. Zhang G, Chen X, Zhang S, et al. Discrimination Between Solitary Brain Metastasis and Glioblastoma Multiforme by Using ADC-Based Texture Analysis: A Comparison of Two Different ROI Placements. Acad Radiol. 2019;26:1466–1472.
3. Schachter M, Does MD, Anderson AW, et al. Measurements of restricted diffusion using an oscillating gradient spin-echo sequence. J Magn Reson. 2000;147:232–237.
Figures
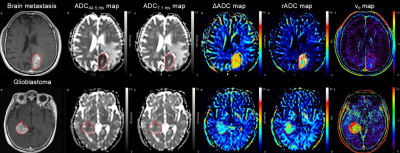
Figure 1. Post-contrast T1-weighted images, diffusion parametric maps, and extravascular extracellular space maps from brain metastasis
(top row) and glioblastoma (bottom row). Note that compared to glioblastoma, brain metastasis exhibits higher values of ΔADC and rADC and lower values of ve.
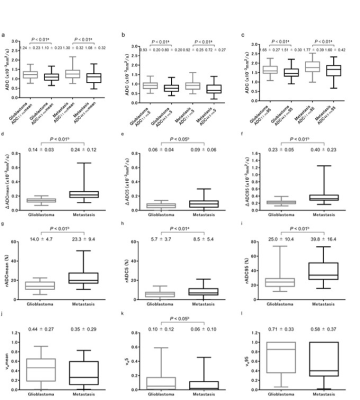
Figure
2. Diffusion parameters and ve in glioblastoma and brain metastasis.
The left column refers to mean values, the middle column to the 5th percentile
and the right column to the 95th percentile of each parameter. Note that brain
metastasis shows significantly
higher ΔADC and rADC indices, and significantly lower ve5
than glioblastoma.
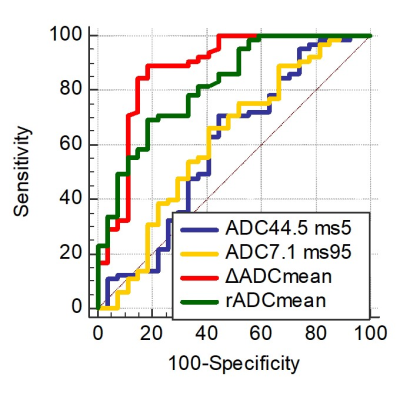
Figure
3. Receiver operating characteristic curve of each parameter for
differentiating brain metastases from glioblastomas (27 brain metastases and 65
glioblastomas). ΔADC: ADC7.1ms - ADC44.5ms; rADC: (ADC7.1ms
- ADC44.5ms)/ADC44.5ms × 100.
DOI: https://doi.org/10.58530/2023/1741