1734
Mapping of neurodegenerative changes with Diffusion Tensor Imaging of Spinocerebellar ataxia type 1, 2 and 12 patients1Nuclear Magnetic Resonance, All India Institute of Medical Sciences, New Delhi, New Delhi, India, 2Neurology, All India Institute of Medical Sciences, New Delhi, New Delhi, India, 3School of Computer & Systems Sciences, Jawaharlal Nehru University, New Delhi- 110 067, New Delhi, India, 4Neuro-radiology, All India Institute of Medical Sciences, New Delhi, New Delhi, India, 5Neuropsychology, All India Institute of Medical Sciences, New Delhi, New Delhi, India
Synopsis
Keywords: Neurodegeneration, Diffusion Tensor Imaging
The study assessed changes in brain tissue microstructures in SCA type 1, 2 and 12 patients using diffusion tensor imaging. Our findings exhibited widespread reduced fractional anisotropy (FA), increased radial (RD) and axial diffusivity (AD) in SCA with respect to that in healthy subjects. FA was decreased in SCA1 and SCA2 in the superior and inferior longitudinal fasciculus, anterior thalamic radiation, inferior fronto-occipital fasiculus, forceps minor, corticospinal tract, cingulum, uncinate fasciculus as compared to controls. Abnormal white matter structure may be linked to cognitive and behavioural impairment in SCA 1 and SCA 2 patients.Introduction
Autosomal dominant Spinocerebellar ataxias (SCAs), one of the most prevalent progressive neurodegenerative ataxias, is an inherited cerebellar disorder1. SCA is characterized by progressive cerebellar ataxia with oculomotor dysfunction, cognitive impairment symptoms pyramidal, and extrapyramidal signs2. Overlapping features of many genetic ataxias can make diagnosis and distinction difficult. Atrophy and abnormalities in the brainstem and cerebellum are reported in SCA patients using MR imaging3. Cortico-ponto-cerebello-thalamo-cortical loops play a role in cognitive functions, and a correlation of the cerebellum with premotor and supplementary motor areas (cortico-cerebello-cortical loops) has also been reported4.SCA2 progression is fast and more severe than other SCAs, resulting in the greatest white matter volume loss compared to other SCAs5. SCA12 progresses slowly and causes late-onset when compared to other SCAs. Clinical neuroradiologic differentiation of SCA1, SCA2 and SCA12 are very difficult6. Diffusion tensor imaging is a quantitative non-invasive method for measuring changes in white matter fiber tracts. Fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD) are used to study the microstructural damage of white matter in SCA1, SCA2 and SCA12 and compare with that in healthy subjects.
Methodology
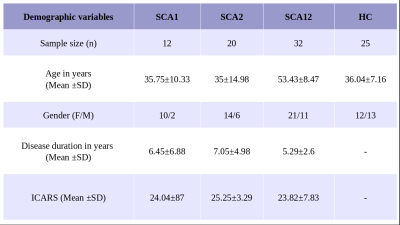
Symptomatic and genetically confirmed 64 SCA (SCA type 1, 2 and 12) patients (SCA1 (n= 12, M/F: 10/2, mean age= 35.75±10.33 years) SCA2 (n=20, M/F:14/6, mean age 35.00±14.98 years), SCA12 (n=32, M/F:21/11, mean age 53.43±8.47 years) patients and healthy controls (HC, n=25, M/F:12/13, mean age 36.04±7.16 years) were recruited after institutional ethical clearance. All SCA patients were assessed (Table 1) with International Cooperative Ataxia Rating Scale (ICARS).MR data was acquired on a 3T MR scanner (Ingenia 3.0 T, M/s. Philips Healthcare, The Netherlands) using 32-channel head coil. Tl weighted 3D Turbo Field Echo (TFE) sequence was used (TR/TE: 8.1 /3.7 ms; flip angle:8°; FOV-240*240; Slices -360 with no gap) as an overlay.
Diffusion tensor imaging (DTI) data were acquired using a single-shot echo-planar dual SE sequence in 32 directions with acquisition parameters: b-factor= 0 and 800 s/mm2, slice thickness=3 mm with no inter-slice space, number of slices=74, FOV=230 mm×230 mm, matrix size = 128 × 128, TR = 4797 ms, EPI factor 39, TE:100ms, flip angle: 90◦, bandwidth: 1502, base resolution: 128, phase resolution: 100, phase encoding direction: A-P.
Data processing
Pre and post-processing of the diffusion tensor images were performed using software tools from the FMRIB software library (FSL, http://www.fmrib.ox.ac.uk/fsl). DTI images were reoriented and then eddy current correction was done. Brain extraction was performed using FSL’s Brain Extraction Tool (BET) and using DTIFIT, fractional anisotropy (FA), radial diffusivity (RD), axial diffusivity (AD) and, mean diffusivity (MD) maps were generated. Tract-Based Spatial Statistics (TBSS) were used to create the mean and mean skeletonized map of FA, MD, RD and AD. Inter-group analyses were performed using one-way ANOVA between the SCA1, SCA2, SCA12 and HC (a p-value <0.05 considered as significant).Result
Bilaterally decreased FA values were observed in SCA1, SCA2 and SCA12 groups in comparison with healthy controls. FA was decreased in SCA1 and SCA2 in the superior and inferior longitudinal fasciculus, anterior thalamic radiation, inferior fronto-occipital fasciculus, forceps minor, corticospinal tract, cingulum, uncinate fasciculus as compared to controls. SCA1 exhibited decreased FA in the left side of corticospinal tract, forcep minor, anterior thalamic radiation, uncinate fasciculus in comparison with SCA12. SCA2 also showed decreased FA in the left cerebellum, vermis and corticospinal tract as compared to SCA12. We also observed increased RD and AD values in SCA1 and SCA2 in bilateral anterior thalamic radiation and corticospinal tract as compared to SCA12. Cerebellar atrophy of white matter was observed in SCA1 and SCA2 in bilateral anterior and posterior lobes with respect to healthy controls. SCA2 patients exhibited more atrophy in comparison to that SCA12.Discussion
Structural imaging revealed atrophy and degeneration in the cerebellum and pons in SCA patients7. Motor control and voluntary activity such as balance, speech and posture are controlled predominantly by cerebellum8. Decreased FA in SCA2 and SCA1 suggest more motor dysfunction in comparison to that in SCA129. Increased AD, RD and decreased FA in the corticospinal tract, superior longitudinal fasciculus, inferior longitudinal fasciculus and anterior thalamic radiation may be attributed to more tremor and disbalace10. Reduced FA in uncinate fasciculus, cingulum and anterior thalamic radiation may be associated with deficits in executive and motor function, emotional and memory impairment11. Diffusivity maps (FA, AD and RD) could be used for discriminating SCA1, SCA2 and SCA12. However, the correlation between white matter fiber strength and the neuropsychological scores of subjects is needed to establish its functional consequence.Conclusion
DTI is useful in differentiating SCA1, SCA2 and SCA12 patients with reference to healthy controls. More atrophy and significantly decreased FA in the cerebellum and corticospinal tract suggest increased motor dysfunction in SCA1 and SCA2 with respect to SCA12. Reduction of FA in major white matter pathways may be associated with cognitive and behavioral impairment.Acknowledgements
We thank the Science and Engineering Research Board (SERB), New Delhi, India for funding [Project No: EMR/2017/002294 & 03rd October 2018]References
1. Klockgether, T., Lu, R., Riess, O., Laccone, F., Kramer, B., Abele, M., Bu, K., Scho, L., Boesch, S., Brice, A., Inzelberg, R., Zilber, N., & Dichgans, J. (1998). The natural history of degenerative ataxia : a retrospective study in 466 patients. 589–600.
2. Choudhury, S., Chatterjee, S., Chatterjee, K., Banerjee, R., Humby, J., Mondal, B., Anand, S. S., Shubham, S., & Kumar, H. (2018). Clinical Characterization of Genetically Diagnosed Cases of Spinocerebellar Ataxia Type 12 from India. Movement Disorders Clinical Practice, 5(1), 39–46.
3. Stezin, A., Bhardwaj, S., Hegde, S., Jain, S., Bharath, R. D., Saini, J., & Pal, P. K. (2021). Cognitive impairment and its neuroimaging correlates in spinocerebellar ataxia 2. Parkinsonism & Related Disorders, 85(November 2020),78-83.
4. Bernard, J. A., Seidler, R. D., Hassevoort, K. M., Benson, B. L., Welsh, R. C., Wiggins, J. L., Jaeggi, S. M., Buschkuehl, M., Monk, C. S., Jonides, J., & Peltier, S. J. (2012). Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Frontiers in Neuroanatomy, 6, 31.
5. Marzi, C., Ciulli, S., Giannelli, M., Ginestroni, A., Tessa, C., Mascalchi, M., & Diciotti, S. (2018). Structural Complexity of the Cerebellum and Cerebral Cortex is Reduced in Spinocerebellar Ataxia Type 2. Journal of Neuroimaging, 28(6), 688–693.
6. Manto, M. and Habas, C., 2016. Cerebellar disorders: clinical/radiologic findings and modern imaging tools. Handbook of Clinical Neurology, 135, pp.479-491.
7. Carass, A., Cuzzocreo, J. L., Han, S., Hernandez-Castillo, C. R., Rasser, P. E., Ganz, M., Beliveau, V., Dolz, J., Ben Ayed, I., Desrosiers, C., Thyreau, B., Romero, J. E., Coupé, P., Manjón, J. V., Fonov, V. S., Collins, D. L., Ying, S. H., Onyike, C. U., Crocetti, D., … Prince, J. L. (2018). Comparing fully automated state-of-the-art cerebellum parcellation from magnetic resonance images. NeuroImage, 183, 150–172.
8. Burciu, R. G., Fritsche, N., Granert, O., Schmitz, L., Spönemann, N., Konczak, J., Theysohn, N., Gerwig, M., van Eimeren, T., & Timmann, D. (2013). Brain changes associated with postural training in patients with cerebellar degeneration: A voxel-based morphometry study. Journal of Neuroscience, 33(10), 4594–4604.
9. Mascalchi, M., Toschi, N., Giannelli, M., Ginestroni, A., Nave, R. Della, Nicolai, E., Bianchi, A., Tessa, C., Salvatore, E., Aiello, M., Soricelli, A., & Diciotti, S. (2015). Progression of microstructural damage in spinocerebellar Ataxia Type 2: A longitudinal DTI study. American Journal of Neuroradiology, 36(6), 1096–1101.
10. Constanzo, J., Dumont, M., Lebel, R., Tremblay, L., Whittingstall, K., Masson-Côté, L., Geha, S., Sarret, P., Lepage, M., Paquette, B., & Descoteaux, M. (2018). Diffusion MRI monitoring of specific structures in the irradiated rat brain. Magnetic Resonance in Medicine, 80(4), 1614–162Ma,
11. Jianhua, Chuanjia Wu, Jing Lei, and Xiaoning Zhang. "Cognitive impairments in patients with spinocerebellar ataxia types 1, 2 and 3 are positively correlated to the clinical severity of ataxia symptoms." International journal of clinical and experimental medicine 7, no. 12 (2014): 5765.
Figures

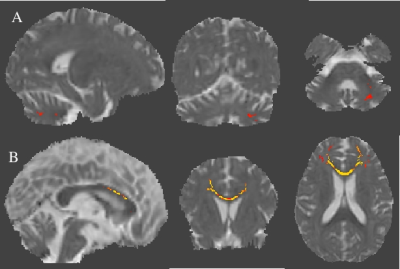
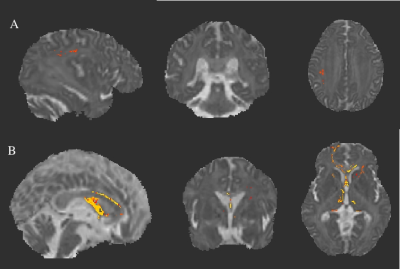
Figure3: Axial diffusivity (AD) maps overlaid on mean AD using Tract-Based Spatial Statistics derived statistics (p<0.05) of Diffusion tensor imaging (DTI) in (A) SCA2 in comparison with SCA12 and (B) SCA1 in comparison with SCA12.