1730
Identifying multimodal imaging biomarkers: a framework exploring association of β-Amyloid Accumulation and Microstructural integrity at 7T1Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Department of Mechanical Engineering, University of Washington, Seattle, WA, United States, 4Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 5Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 6Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Keywords: Neurodegeneration, Diffusion Tensor Imaging, High-Field MRI
Here we outline a preliminary method using a novel method which leverages UHF neuroimaging to measure detectable correlations in two measures: microstructural (mean diffusivity and fractional anisotropy) acquired at 7T and amyloid beta (β-amyloid) accumulation, acquired using 3T PET-MR.The combination of these methods aids in the ability to map tissue structure and achieve unprecedented visualization of the consequences of β-amyloid accumulation as it relates to neurodegenerative disorders. We show the feasibility of leveraging high resolution diffusion to advance out understand of the relationship between fractional anisotropy and mean diffusivity within brain regions that may be affected by β-amyloid deposition.Introduction
Progressive neurodegenerative conditions such as Alzheimer’s disease (AD), the leading cause of dementia have an immense impact on the aging population worldwide(1). AD has a long preclinical phase, and accumulation of beta-Amyloid (Aβ) plaques are believed to be one of the main pathogenic biomarkers of progressive neurodegeneration(2). β-amyloid can be detected through the utility of positron emission tomography (PET) with aid of a radioactive tracer or within cerebral spinal fluid acquired through a lumbar puncture. Since these two procedures are considered invasive, there is a profound need to identify non-invasive correlates of AD pathologies to aid in early detection or diagnosis, ultimately assisting in improved disease management or prevention. Diffusion tensor magnetic resonance imaging (DTI) is an MRI technique that can probe neural microstructure by measuring diffusion of water molecules and provide a metric for understanding the integrity of microstructure(3).Leveraging non-invasive tools such as ultra-high field (UHF), 7 Tesla (7T) MRI, with increased signal-to-noise ratio (SNR) and improved soft tissue contrast due to the higher magnetic field strength allows to map tissue microstructure (mean diffusivity (MD), and fractional anisotropy (FA)) in relation to Aβ accumulation(4) . 7T MRI enables better visualization and microstructure quantification within small regions where visualization may be limited due to size, proximity to sinuses or ventricles, inhomogeneity, signal dropout, and visualization compared to lower field strengths. Additionally, through increased SNR, 7T MRI provides increased accuracy of microstructural integrity estimates such as FA compared to lower field strength counterparts(5). Here we outline a method for leveraging UHF MRI and PET to better understand the progression of β-amyloid accumulation and the consequential effects on underlying microstructure.Methods
An exploratory analysis was performed in 6 amyloid-negative adults (4 females, 2 males, average age of 73.2 ± 5.3 years) with no current or lifetime history of cognitive impairment. Subjects underwent a 7T MRI scan and PET-MR to measure Aβ burden using F18-labeled florbetaben tracer (Table 1). PET attenuation-corrected data from 90–110-minutes post-injection were measured in 1-minute intervals and motion corrected then coregistered to the 3T-T1. The DMRI series in both anterior-to-posterior and posterior-to-anterior directions were collated into a single volume which was denoised and corrected for eddy current distortions, motion, and B1 inhomogeneity using MRtrix3. Whole brain tractography was generated with 10,000 seeds using tractseg. All 7T T1 images were segmented using FreeSurfer 7.2 hi-res6 and masks were generated for each subject: cerebellar, frontal, parietal, and temporal cortex, hippocampus, and entorhinal cortex and left/right per region. For workflow see Figure 1. Preliminary analyses were done using python3 to assess the relationship between β-amyloid deposition and regional FA and MD values(Table 2). Correlation coefficients were calculated using Pearson’s R. Significant relationships between metrics were calculated using linear regression.Results
We provide proof-of-concept of a preliminary method that leverages UHF neuroimaging and PET to explore correlations between underlying microstructure of brain regions which effected by Aβ accumulation. We found significant positive association between Aβ and temporal lobe MD(R=0.939, p=0.0054)and negative association between Aβ and parietal lobe FA(R=-0.844, p=0.035)and left parietal FA(R=-0.944, p=0.005)(Figure 2).Additionally, we found that other regions such as the hippocampus and right entorhinal were trending towards statistically significant(Table 3).Discussion
In this exploratory analysis, we have established a pipeline for potential deeper understanding of the relationship between regional Aβ accumulation and underlying microstructure. By leveraging enhanced SNR through 7T we can derive greater certainty in diffusion metrics and extract microstructural information from more granular regions compared to lower field strengths(7). By mapping tractography within regions of interest onto regions with high amyloid deposition, we may be able to leverage decreases in uncertainty of microstructural integrity estimates to perform a group-level voxel wise analysis to map the overlaps between reductions/increases in diffusion metrics as they relate to region-specific Aβ accumulations.Here we have investigated associations between a known biomarker for aging and neurodegeneration, Aβ, plaques and measures for microstructural integrity (MD and FA) in an exploratory group of amyloid-negative adults. Normal microstructural dysfunction is characterized by decreases in FA and increases in MD. As amyloid load increased, we saw significant reductions in FA values within the parietal lobe, and in parallel, MD values increased within the temporal lobe. This could indicate that as Aβ load increases, that microstructural organization decreases. These microstructural changes may further relate to cognition(8) or underlying pathologies that contribute to progressive neurodegeneration. While these results are preliminary, and sample size may not provide enough power to detect robust effect sizes, with a larger, amyloid-positive sample, we may be able to feasibly leverages high resolution diffusion and extracts regional values that may relate to AD pathology with this method. This may result in changes in association between Aβ and microstructure. We hope to explore this relationship further with recruitment of more subjects including AD patients to develop novel insights in our understanding of the mechanisms and consequences of β-amyloid accumulation as it related to AD progression.
Conclusion
Here we provided a method which leverages UHF neuroimaging and PET to explore correlations between underlying microstructure of brain regions which effected by Aβ accumulation. We have shown a novel approach to achieving unprecedented visualization of pathologies that underlie neurodegeneration and AD progression through UHF neuroimaging.Acknowledgements
This work was funded by: R21AG071179-01 and P30AG066514References
1. Burrinha, T., Gomes, R., Terrasso, A. P., & Almeida, C. G. (2019). Neuronal aging potentiates beta-amyloid generation via amyloid precursor protein endocytosis. Cold Spring Harbor Laboratory. https://dx.doi.org/10.1101/616540
2. Tarawneh, R., & Holtzman, D. M. (2012). The Clinical Problem of Symptomatic Alzheimer Disease and Mild Cognitive Impairment. Cold Spring Harbor Perspectives in Medicine, 2(5), a006148-a006148. https://doi.org/10.1101/cshperspect.a006148
3.Polders, D. L., Leemans, A., Hendrikse, J., Donahue, M. J., Luijten, P. R., & Hoogduin, J. M. (2011). Signal to noise ratio and uncertainty in diffusion tensor imaging at 1.5, 3.0, and 7.0 Tesla. Journal of Magnetic Resonance Imaging, 33(6), 1456-1463. https://doi.org/10.1002/jmri.22554
4. Rabin, J. S., Perea, R. D., Buckley, R. F., Neal, T. E., Buckner, R. L., Johnson, K. A., Sperling, R. A., & Hedden, T. (2019). Global White Matter Diffusion Characteristics Predict Longitudinal Cognitive Change Independently of Amyloid Status in Clinically Normal Older Adults. Cerebral Cortex, 29(3), 1251-1262. https://doi.org/10.1093/cercor/bhy031
5. O’Donnell, L. J., & Westin, C.-F. (2011). An Introduction to Diffusion Tensor Image Analysis. Neurosurgery Clinics of North America, 22(2), 185-196. https://doi.org/10.1016/j.nec.2010.12.004
6. Fischl, B., Liu, A., & Dale, A. M. (2001). Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE transactions on medical imaging, 20(1), 70-80.
7. Rutland, J. W., Feldman, R. E., Delman, B. N., Panov, F., Fields, M. C., Marcuse, L. V., Hof, P. R., Lin, H.-M., & Balchandani, P. (2018). Subfield-specific tractography of the hippocampus in epilepsy patients at 7 Tesla. Seizure, 62, 3-10. https://doi.org/10.1016/j.seizure.2018.09.005
8. Vernooij, M. W., Ikram, M. A., Vrooman, H. A., Wielopolski, P. A., Krestin, G. P., Hofman, A., Niessen, W. J., Van Der Lugt, A., & Breteler, M. M. B. (2009). White Matter Microstructural Integrity and Cognitive Function in a General Elderly Population. Archives of General Psychiatry, 66(5), 545. https://doi.org/10.1001/archgenpsychiatry.2009.5
Figures

Table 1: Imaging parameters for 7T MR acquisition and 3T PET-MR. (Aβ burden was measured with F18–labeled florbetaben simultaneous positron emission tomography and magnetic resonance (PET-MR) imaging on a Siemens Biograph mMR using standardized protocols in accordance with FDA labeling instructions.)
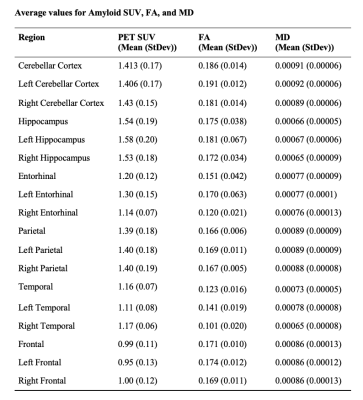
Table 2: Average SUV, Fractional Anisotropy, and Mean Diffusivity for each Brain Region.


