1721
Interaction of Alzheimer’s Disease Status and History of Traumatic Brain Injury on Measures of Cortical Thickness1Institute of Medical Science, University of Toronto, Toronto, ON, Canada, 2Keenan Centre for Biomedical Research, Li Ka Shing Knowledge Institute, St Michael's Hospital, Toronto, ON, Canada, 3Neuroscience Research Program, St Michael's Hospital, Toronto, ON, Canada, 4Physics Department, Toronto Metropolitan University, Toronto, ON, Canada, 5Cumming School of Medicine, University of Calgary, Calgary, AB, Canada, 6Department of Psychiatry, Faculty of Medicine, University of Toronto, Toronto, ON, Canada, 7Division of Neurosurgery, Department of Surgery, Faculty of Medicine, University of Toronto, Toronto, ON, Canada, 8The Institute of Biomaterials & Biomedical Engineering, University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: Alzheimer's Disease, Traumatic brain injury
Traumatic Brain Injury (TBI) is associated with an accelerated course of dementia, although biological relationships are still incompletely understood. We characterized the differences in cortical thickness, for Alzheimer’s Disease (AD) patients with and without a history of TBI. Among individuals diagnosed with AD, a history of TBI was associated with a smaller decrease in cortical thickness in frontal-temporal regions, relative to their non-AD counterparts, with analyses controlling for the effects of age, sex, and education. TBI may lower the susceptibility threshold for cognitive decline related to AD by decreasing an individuals’ ability to cope with aging and/or AD pathology.Introduction
There are over 50 million people living with dementia worldwide, with Alzheimer’s disease (AD) being the most common diagnosis.1 Therefore, it is critical to identify factors that contribute to AD and accelerate the disease course, in order to identify high-risk individuals for improved management and potential point of intervention for new therapeutics. Traumatic brain injury (TBI) is a well-established environmental risk factor for AD and other dementias.2-5 Several epidemiological studies have indicated that a history of TBI is associated with an increased risk of dementia and a faster course of disease progression.6-8 Based on TBI incidence and associated risk of dementia, it has been estimated that 5-15% of all AD cases may be attributable to TBI.9 Given the high prevalence of TBI, establishing a clear link with AD would have major implications for early risk assessment and preventative intervention; but biological relationships remain incompletely understood.Magnetic resonance imaging (MRI) techniques have the potential to reveal distinct and informative patterns of altered grey matter morphology in AD and TBI. Cortical thickness in particular, may be decreased in neurodegenerative disorders as a consequence of multiple upstream neuropathological changes.10 Structural imaging studies of cortical thickness in patients with AD have demonstrated atrophy in medial temporal and posterior temporo-parietal regions.11,12 Studies examining the long-term effects of TBI have also found cortical thinning in fronto-temporal regions.13,14 These findings indicate partial overlap of TBI-related regions and those identified in the AD literature. At present, however, our understanding of the extent to which AD and TBI share patterns of neurodegeneration is limited by the lack of direct studies combining these groups. Most of the TBI literature focus on young adults in athlete and military cohorts, whereas AD studies focus on older cohorts drawn from the general population, requiring that we make indirect inferences across different demographic cohorts. It is therefore critical to directly investigate the combined effects of AD and prior TBI within normal aging cohorts.
Objectives
The goal of the current study was to examine cortical thickness of AD patients with and without history of TBI, along with a group of controls with and without history of TBI, to identify the independent effects of AD and TBI on cortical thickness, along with the moderating effect of TBI on AD. This was examined cross-sectionally within the frontal and temporal lobes, areas that consistently exhibit AD pathology and are vulnerable to TBI effects.13-15Methods
The study examined a large cohort of 1124 participants from the National Alzheimer’s Coordinating Center (NACC) database. Participants included were clinically diagnosed with AD and reported a history of TBI (n=127 with AD+TBI), clinically diagnosed with AD without a history of TBI (n= 343 with AD), cognitively normal adults without TBI (n=388 CN) or cognitively normal adults with TBI history (n=266 with TBI). T1-weighted MRI scans were collected, and cortical thickness quantified using FreeSurfer segmentation of the frontal and temporal lobes. Cross-sectional analyses employed multiple linear regression models to assess the association of baseline cortical thickness in frontal and temporal regions of interest with TBI status and AD status, for each cortical region of interest.Results
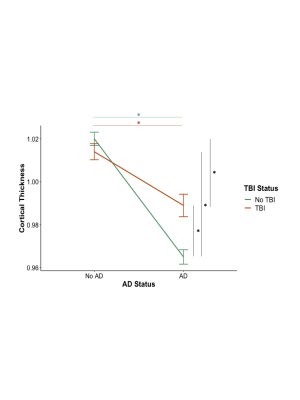
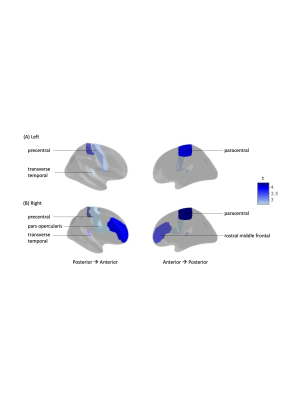
Among individuals diagnosed with AD, a history of TBI was associated with a smaller decrease in cortical thickness, relative to their non-AD counterparts, with analyses controlling for the effects of age, sex, and education (Figure 1). This was seen in fronto-temporal regions including the paracentral, precentral, pars opercularis, rostral middle frontal, and transverse temporal (Figure 2).Conclusions
In this study, we characterized the differences in cortical thickness, for AD patients with and without a history of TBI, using a large sample drawn from the NACC database. The present results provide insight on AD-related brain changes that may be altered by a history of TBI, suggesting that TBI lowers the susceptibility threshold for cognitive decline related to AD by decreasing an individuals’ ability to cope with aging and/or AD pathology and accelerate symptom onset. Our findings have important implications on the diagnosis of TBI-related dementia and development of targeted treatments.Acknowledgements
The authors acknowledge NACC for their support of this project. The NACC database is funded by NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA-funded Alzheimer's Disease Research Centers (ADRCs).References
1. World Health Organization. Dementia. World Health Organization; 2022.
2. Wang H-K., Lin SH, Sung P-S, et al. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry. 2012;83(11):1080-1085.
3. Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55(8):1158-1166.
4. LoBue C, Wadsworth H, Wilmoth K, et al. Traumatic brain injury history is associated with earlier age of onset of Alzheimer disease. The Clinical Neuropsychologist. 2017;31(1):85-98.
5. Barnes DE, Kaup A, Kirby KA, et al. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83(4):312-319.
6. Schaffert J, LoBue C, White CL, et al. Traumatic brain injury history is associated with an earlier age of dementia onset in autopsy-confirmed Alzheimer’s disease. Neuropsychology. 2018;32(4):410-416.
7. Li Y, Li Y, Li X, et al. Head injury as a risk factor for dementia and Alzheimer’s disease: a systematic review and meta-analysis of 32 observational studies. PloS one. 2017;12(1):e0169650.
8. Nordström A and Nordström P. Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PloS Medicine. 2018;15(1):e1002496.
9. Graham NSN and Sharp DJ. Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry. 2019;90(11):1221-1233.
10. Schwarz CG, Gunter JL, Wiste HJ, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage: Clinical. 2016;11:802-812.
11. Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer's Disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral Cortex. 2009;19(3):497-510.
12. Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: Regional thinning predicts mild AD dementia. Neurology. 2009;72(12):1048-1055.
13. Churchill N, Hutchison M, Richards D, et al. Brain structure and function associated with a history of sport concussion: a multi-modal magnetic resonance imaging study. J Neurotrauma. 2017;34(4):765-771.
14. Mazaharally M, Stojanovski S, Trossman R, et al. Patterns of change in cortical morphometry following traumatic brain injury in adults. Human Brain Mapping. 2022;43(6):1882-1894.
15. Sabuncu MR, Desikan RS, Sepulcre J, et al. The Dynamics of Cortical and Hippocampal Atrophy in Alzheimer’s Disease. Arch Neurol. 2011;68(8):1040-1048.
Figures