1720
Atrophy patterns of hippocampal subregions based on automated volumetry in Parkinson's disease with type 2 diabetes mellitus patients1The First Affiliated Hospital of Dalian Medical University, Dalian, China, 2GE Healthcare, Shanghai, China
Synopsis
Keywords: Nerves, Diabetes
The objective of this study was to evaluate the atrophy pattern of the hippocampus subregions in Parkinson's disease with type 2 diabetes mellitus patients (PDDM) patients based on the 3DTI sequence and FreeSurfer software. The FreeSurfer provides extensive and automated neuroimaging analysis and has been successfully used to precisely segment the hippocampus. In this study, automated volumetry of hippocampal subfields in PDDM patients was performed, compared with Parkinson's disease without diabetes mellitus (PDND) patients and healthy controls . The results showed that PDDM patients have hippocampal subregions atrophy, especially in the right fimbria, right GC-ML-DG and right CA4.Summary of Main Findings
In this study, automated volumetry of hippocampal subregions in Parkinson's disease with type 2 diabetes mellitus patients (PDDM) patients was performed, compared with Parkinson's disease without diabetes mellitus (PDND) patients and healthy controls. The results showed that PDDM patients have hippocampal subregions atrophy, especially in the right fimbria, right GC-DG and right CA4.Introduction
Type 2 diabetes (T2DM) and Parkinson's disease (PD) are prevalent diseases that affect an aging population. There is increasing evidence for shared biology between T2DM and PD. Both are characterized by aberrant protein accumulation, lysosomal and mitochondrial dysfunction, and chronic systemic inflammation[1]. Insulin resistance is a hallmark of T2DM and may also be an important contributing factor to PD[2]. In PD patients, cognitive impairment is related to changes in hippocampal structure and function. Moreover, the hippocampus is very sensitive to T2DM. In the hippocampus, insulin receptor and IGF-1 receptor are widely expressed, mainly mediating cognitive functions such as spatial learning and memory[3]. To date, how T2DM affects hippocampal subregion volumes in PD is unclear. In this study, we aim to use an automatic hippocampal segmentation method to determine if there was a change in subfield hippocampal volume in PDDM patients.Materials and Methods
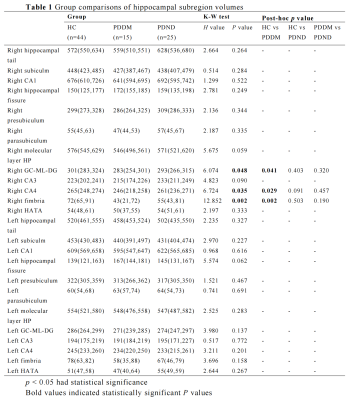
A total of 84 individuals were retrospectively recruited, including PDDM patients (n = 15), PDND patients (n = 25) and HC (n = 44). All Participants were scanned using a 3.0 T GE Signa HDXT scanner from America equipped with an 8-channel head coil. A 3D magnetization-prepared rapid-acquisition gradient-echo T1-weighted sequence with the following parameters was performed: repetition time = 10.2 ms, echo time (shortest) = 4.2 ms, flip angle = 13°, FOV = 24 × 24cm2, matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm, slice thickness 1.0 mm. Automated volumetric measurement of the hippocampal subregions was done using FreeSurfer 6.0 hippocampal subregions module. The hippocampal was segmented, bilaterally, into the hippocampal tail, subiculum, CA1, hippocampal fissure, presubiculum, parasubiculum, molecular layer, GC-ML-DG (granule cell and molecular layer of the DG), CA3, CA4, fimbria and hippocampus amygdala transition area (HATA). The estimated total intracranial volume (eTIV) volumes were also extracted. Data analyses were performed using SPSS 26.0, the Kruskal-Wallis test was used to compare the hippocampal subregion volumes among the three groups, and then Post hoc pairwise comparisons were done by Bonferroni correction. P < 0.05 was considered statistically significant.Result
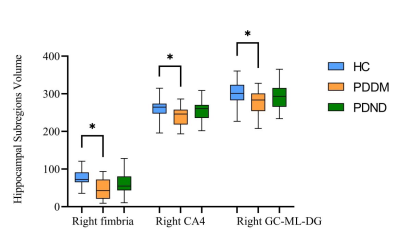
Significant volumetric differences in the three groups were found for right fimbria (p = 0.002), right GC-ML-DG (p = 0.048) and right CA4 (p = 0.035, Table 1). Then we performed post-hoc analyses in these three hippocampal subregions. All three hippocampal subregions showed a significantly lower volume in the PDDM group than in the NC group (p < 0.05, Figure 1). There were no statistically significant differences in the other subregions among these three groups.Discussion
This study explored the atrophy pattern of hippocampal subregions in PDDM patients. We observed the reduced volumes of right fimbria, right GC-DG and right CA4 in patients with PDDM while compared with HCs. The fimbria is a white matter structure related to visuospatial function and object discrimination[4]. Insulin and its receptors are widely expressed in the brain and play a critical role in neuronal proliferation and differentiation. The subgranular zone, located in the dentate gyrus of the hippocampus, is one of the two major neural stem cell regions[5]. A study showed that entorhinal cortex stimulation promotes neurogenesis in the hippocampal DG region of adult rats, while insulin receptor antagonists attenuated neurogenesis[6]. Therefore, we hypothesized that insulin resistance might weaken neurogenesis in the dentate gyrus of the hippocampus, leading to the decrease in the volumes of the hippocampal subregions. CA4 is located in the dentate gyrus and can receive excitation from the cerebral cortex, so the loss of synapses in these two regions affects the information input of the hippocampus.Conclusion
We observed reduced hippocampal subregion volumes in PDDM patients, mainly in the right hippocampal subregions, which were in the right fimbria, right GC-ML-DG and right CA4. We speculate that insulin resistance may partially affect the atrophy of the hippocampal subfield volumes, which may impair neurogenesis in the hippocampal dentate gyrus.Acknowledgements
No acknowledgement found.References
[1] Cheong JLY, de Pablo-Fernandez E, Foltynie T, Noyce AJ. The Association Between Type 2 Diabetes Mellitus and Parkinson's Disease. J Parkinsons Dis. 2020;10(3):775-789.
[2] Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2011 Sep 13;8(2):92-103.
[3] Soto M, Cai W, Konishi M, Kahn CR. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc Natl Acad Sci U S A. 2019 Mar 26;116(13):6379-6384.
[4] Foo H, Mak E, Chander RJ, Ng A, Au WL, Sitoh YY, Tan LC, Kandiah N. Associations of hippocampal subfields in the progression of cognitive decline related to Parkinson's disease. Neuroimage Clin. 2016 Dec 12;14:37-42.
[5] Farrar C, Houser CR, Clarke S. Activation of the PI3K/Akt signal transduction pathway and increased levels of insulin receptor in protein repair-deficient mice. Aging Cell. 2005 Feb;4(1):1-12.
[6] Lee CC, Huang CC, Hsu KS. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011 Sep;61(4):867-79.