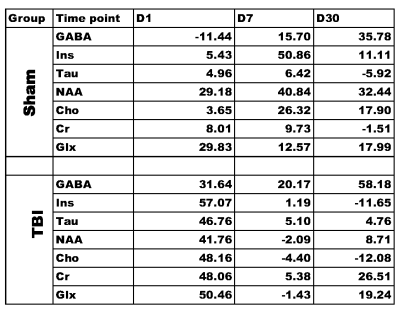
1713
Characterization of a controlled cortical impact brain injury and metabolic changes using magnetic resonance spectroscopy1Georgia medical College, Augusta, GA, United States, 2Georgia Cancer Center, Augusta University, Augusta, GA, United States, 3Neurosurgery, Augusta University, Augusta, GA, United States
Synopsis
Keywords: Traumatic brain injury, Spectroscopy
Traumatic brain injury (TBI) diagnosis and treatment is a top priority for military medicine. Several preclinical models of TBI have been developed to help elucidate the etiology of brain injury but these models typically depend on behavioral outcomes to indicate injury severity. In this project we used magnetic resonance spectroscopy (MRS) to investigate a noninvasive, longitudinal technique to detect changes in brain metabolites after TBI
Introduction
Severe traumatic brain injury (TBI) induces immediate axonal injury1,2, yet the majority of neurodegeneration, including bilateral matter (WM) tract loss, develops over months and years after injury. Cellular, immune and hormonal cascades occurring after injury and continuing during the healing process may impact uninjured brain regions sensitive to the effects of physiological and emotional stress, which receive projections from the injury site. Changes in these most basic properties due to injury or disease have profound implications for virtually every aspect of brain function through disruption of neurotransmitter, neuroendocrine and metabolic systems. In order to screen for changes in transmitter and metabolic activity, in this research we analyzed changes in metabolites from traumatic penumbra region of brain in mice following TBI/sham using proton Magnetic Resonance Spectroscopy (1H-MRS).Methods
C57BL/7 mice were injured by CCI at 8-10 weeks old. Sham animals underwent anesthesia and scalp incision without a craniectomy or injury. Each mouse was scanned four times, baseline (a day before injury), 1, 3 and 30 days following TBI. In-vivo single voxel 1H-MRS was performed on a Bruker BioSpec system (Bruker NMR, Inc., Billerica, MA) consisting of a 7-Tesla (T), 20-cm horizontal bore, superconducting magnet (Magnex Scientific, Abingdon UK) with a Biospec 70/20 console and Paravision software. An Autopac mouse positioning and physiological monitoring system, 86mm quadrature transmit coil and a 4-channel phase array mouse head coil were used. Mice were anesthetized with isoflurane during the preparation period and during the scan using a ‘flow through’ nose cone (1.5-2.0%). For Single voxel 1H-MRS, the volume of interest (VOI, 2mm x 2mm x 2mm) was placed over the traumatic penumbra region in the coronal, axial and sagittal T2 weighted images before. The data was acquired using Point RESolved Spectroscopy (PRESS, TR/TE = 2500/20ms, averages = 256), with and without water suppression, localized at the contralateral and ipsilateral optical track. The scan time for MR imaging and spectroscopic experiment was approximately 2 hours for each time point. Spectra were processed in both Tarquin and LC-Model (S.W. Provencher), with eddy current correction and water-scaling, to quantify brain metabolites N-acetylaspartate (NAA), glutamate+glutamine (Glx), choline (Cho), creatine (Cr) and myo-inositol (mI) in the selected regions. Concentrations between groups were statistically analyzed in MATLAB (The Math Works, Natick, MA) and GraphPad (GraphPad, La Jolla, CA) for significance.Results and Discussion
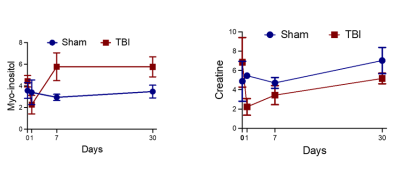
The anatomic images along with the voxel locations in the ipsilateral region is shown in figure 1. The spectra demonstrate excellent sensitivity at both contralateral and ipsilateral sides. Table 1 shows the percentage difference between the baseline acting as control and different time point. Figure 2 shows the metabolic changes for Myo-inositol at day 7 and 30 with significant difference between sham and TBI group. However this this difference wash out if we normalized each animal with the contralateral side. This indicate that the TBI affect the metabolic changes in the whole brain and not only in traumatic penumbra region of brain where cell are believe to be changing. Figure 3 Glx shows significant decrease in day 1 compare to both baseline and day 7. This decrease level of Glx require future investigation. Future studies using these animals will be important for the understanding the functional role of these metabolites in the injured brain and it involvement in the long term neurological and mental disorder.Summary
This study focused on 1H-MRS findings in injured brain with sham animals. These changes in metabolites in the brain after severe CCI in mice needs to be studied at a later time point to see its effect on behavior outcome. The TBI group had reduction in all metabolites in acute stage D1 but the sham group did not. There seems to be recovery of metabolites chronic stage compare to the baseline for the TBI. This need further investigation to look at these neural makers for stress due to the TBI for future studies and recovery after TBIAcknowledgements
The work was supported by funds from Internal grants at Georgia Cancer Center, Augusta University and NIH R01 NS110378 and R01 NS117565 Dhandapani (PI)References
1. Buki A, Povlishock JT. All roads lead to disconnection?--Traumatic axonal injury revisited. Acta Neurochir (Wien). 2006;148:181-193; discussion 193-184. doi: 10.1007/s00701-005-0674-4
2. Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35-43. doi: 10.1016/j.expneurol.2012.01.013 white matter (WM) tract loss, develops over months and years
3. Provencher SW (2001) Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14: 260-264
4. Kossowski B, Orzel J, Bogorodzki P, Wilson M, Setkowicz Z et al. (2017) Follow-up analyses on the effects of long-term use of high fat diet on hippocampal metabolite concentrations in Wistar rats: Comparing Tarquin quantification of 7.0T rat metabolites to LCModel. Biol Eng Med 2: DOI: 10.15761/BEM.1000129
Figures