1704
Assessment of Glx and GABA levels in primary dysmenorrhea patients in menstruation and periovulatory phases1Department of Radiology, The First Affiliated Hospital of Soochow University, Suzhou, China, 2Philips Healthcare, Shanghai, China, 3Institute of Medical Imaging, Soochow University, Suzhou, China, 4National Clinical Research Center for Hematologic Diseases, The First Affiliated Hospital of Soochow University, Suzhou, China
Synopsis
Keywords: Nerves, Brain
The pathogenesis of primary dysmenorrhea (PDM) and the central nervous system (CNS) mechanisms leading to poorer mode and pain sensitization remain totally unclear, which was explored in our study using MEGA-PRESS. PDM patients showed significantly higher Glx level and mildly higher GABA+ level (not significantly) in ACC in menstruation phase. In menstruation phase, the SDS/PCS scores of the PDM patients had a positive correlation with ACC GABA+ levels. The imbalances in ACC GABA+/Glx levels in PDM patients in menstruation phase may be the mechanism mediating depressive symptoms and pain catastrophe.
Keywords
1H-MRS, MEGA-PRESS, Primary dysmenorrhea, Gamma-aminobutyric acid, Glutamate/glutamine, Anterior cingulate cortexIntroduction
Primary dysmenorrhea (PDM) is a common gynecological condition that affects 45%~95% of menstruating women and at least 33% females report moderate or severe menstrual pain, which negatively impacts on their quality of life1-4.The central nervous system (CNS) mechanisms leading to poorer mode and pain sensitization remain totally unknown. The anterior cingulate cortex (ACC) is particularly important for pain and unpleasantness with negative mood. Monthly painful episodes induced greater sensitivity in the CNS both in painful and pain-free states5-7. Dysfunction of neurochemical metabolism is supposed to be responsible for altered pain processing and manifestation of chronic pain8 9. However, the specific levels of gamma-aminobutyric acid (GABA) and glutamate/glutamine (Glx) neurotransmitter levels in the ACC and their relationships with the clinical characteristics have not been researched in PDM patients. The purpose of our study was to apply 1H MEGA-PRESS spectroscopy to quantify ACC neurotransmitters in PDM patients and their age- and education-matched healthy controls (HCs) in both menstruation and periovulatory phases and to test whether the neurotransmitter concentrations are related to clinical characteristics.Methods
Finally, 41 PDM participants and 39 HC participants were included in the study (Figure 1). Each participant underwent menstruation and periovulatory phase MRI scans. Self-rating anxiety scale (SAS), self-rating distress scale (SDS), Visual Analogue Scale (VAS) and Pain Catastrophizing Scale (PCS) were assessed before each MRI scanning. Imaging was performed on a Philips Ingenia 3.0 Tesla MR scanner (Philips Healthcare, Best, Netherlands) with a fifteen-channel phased-array head coil to obtain the single-voxel 1H-MEGE-PRESS MRS with the following parameters: TR/TE = 2000/68 ms; number of points = 2048; spectral bandwidth = 2000 Hz; number of averages/unsuppressed water averages = 320/8. The voxel of the 1H-MRS in the ACC region was a size of 4×2×3 cm3 (24cm3). The water and Cr peaks were both applied as reference signals to measure the reliability and repeatability of metabolite data. The full-width half-maximum (FWHM) of reference signals higher 10 Hz and data with a fit error of higher 15% were excluded. The detailed process is shown in Figure 2. The GABA+ and Glx levels in the ACC were compared between groups and between two phases in each group, respectively. The GABA+ and Glx levels of PDMs were correlated with clinical characteristics.Results
PDM patients showed higher SAS, SDS and PCS scores in both menstruation and periovulatory phases (p<0.05). Compared to HCs, PDMs showed significantly higher Glx levels (pCr referencing = 0.003; pWater referencing = 0.002; pCSF−corrected = 0.004) and mildly higher GABA+ levels (not significantly p>0.05) in the ACC in menstruation phase (Table 1). PDMs themselves had the same neurotransmitter contents tread in menstruation phase when compared to periovulatory phase (Table 2). In menstruation phase, the SDS/PCS scores of the 34 PDM patients had positive correlations with the GABA+ levels (rCr referencing = 0.369, pCr referencing = 0.045/rCr referencing = 0.373, pCr referencing = 0.042; rWater referencing = 0.364, pWater referencing = 0.048) (Figure 3). There was no significant correlation in periovulatory phase in each group. All the correlation analysis were adjusted for age, education level, migraine, and family history of dysmenorrhea.Discussion
The interpretation of ACC increased Glx levels with mildly higher GABA+ concertation changed in menstruation phase in PDMs here is complex. The imbalance in the glutamate/GABAergic system could be present in a wide range of disorders and supposed to be responsible for altered pain processing and manifestation of chronic pain9 14. The plastic changes of astrocytes in respond to the repeated menstrual pain may eventually result in the hyperexcitability of ACC glutamate levels and thus imbalance in the glutamate/GABAergic, which may eventually result in the mildly higher GABA levels. For the PDM patients themselves, one menstrual pain may be seemed like a kind of acute pain compared to the pain-free state. The results of higher Glx concentration were partly in line with findings of previous acute pain studies15-17. Long-term chronic pain leads to abnormal reconstruction of brain, which may further affect psychological self-regulation and make patients more distressed18. There were positive relationships between a mildly higher tread of GABA+ levels and SDS/PCS scores in PDMs in menstruation phase. This may support the involvement of ACC-GABAergic neurotransmission in cognitive function and motivational pain medication.Conclusion
Our results suggest that the imbalances in the GABAergic and glutamatergic system in ACC may be a neurochemical association with negative mode and pain catastrophic in PDM.Acknowledgements
No acknowledgement found.
References
1. Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Human Reproduction Update2015;21(6):762-78. doi: 10.1093/humupd/dmv039
2. Burnett M, Lemyre M. No. 345-Primary Dysmenorrhea Consensus Guideline. J Obstet Gynaecol Can 2017;39(7):585-95. doi: 10.1016/j.jogc.2016.12.023 [published Online First: 2017/06/20]
3. Ferries-Rowe E, Corey E, Archer JS. Primary Dysmenorrhea: Diagnosis and Therapy. Obstet Gynecol 2020;136(5):1047-58. doi: 10.1097/aog.0000000000004096 [published Online First: 2020/10/09]
4. Hu Z, Tang L, Chen L, et al. Prevalence and Risk Factors Associated with Primary Dysmenorrhea among Chinese Female University Students: A Cross-sectional Study. J Pediatr Adolesc Gynecol 2020;33(1):15-22. doi: 10.1016/j.jpag.2019.09.004 [published Online First: 2019/09/21]
5. Dawood MY. Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol 2006;108(2):428-41. doi: 10.1097/01.AOG.0000230214.26638.0c [published Online First: 2006/08/02]
6. Low I, Wei SY, Lee PS, et al. Neuroimaging Studies of Primary Dysmenorrhea. Adv Exp Med Biol 2018;1099:179-99. doi: 10.1007/978-981-13-1756-9_16 [published Online First: 2018/10/12]
7. Tu CH, Niddam DM, Chao HT, et al. Brain morphological changes associated with cyclic menstrual pain. Pain 2010;150(3):462-68. doi: 10.1016/j.pain.2010.05.026
8. White TL, Monnig MA, Walsh EG, et al. Psychostimulant drug effects on glutamate, Glx, and creatine in the anterior cingulate cortex and subjective response in healthy humans. Neuropsychopharmacology 2018;43(7):1498-509. doi: 10.1038/s41386-018-0027-7 [published Online First: 2018/03/08]
9. Zhuo M. Cortical excitation and chronic pain. Trends in neurosciences 2008;31(4):199-207. doi: 10.1016/j.tins.2008.01.003 [published Online First: 2008/03/11]
10. Edden RA, Puts NA, Harris AD, et al. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging 2014;40(6):1445-52. doi: 10.1002/jmri.24478 [published Online First: 2014/12/31]
11. Srinivasan R, Sailasuta N, Hurd R, et al. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 2005;128(Pt 5):1016-25. doi: 10.1093/brain/awh467 [published Online First: 2005/03/11]
12. Shungu DC, Mao X, Gonzales R, et al. Brain γ-aminobutyric acid (GABA) detection in vivo with the J-editing (1) H MRS technique: a comprehensive methodological evaluation of sensitivity enhancement, macromolecule contamination and test-retest reliability. NMR Biomed 2016;29(7):932-42. doi: 10.1002/nbm.3539 [published Online First: 2016/05/14]
13. Arm J, Oeltzschner G, Al-Iedani O, et al. Altered in vivo brain GABA and glutamate levels are associated with multiple sclerosis central fatigue. Eur J Radiol 2021;137:109610. doi: 10.1016/j.ejrad.2021.109610 [published Online First: 2021/03/04]
14. Zugaib J, Coutinho MR, Ferreira MD, et al. Glutamate/GABA balance in ACC modulates the nociceptive responses of vocalization: an expression of affective-motivational component of pain in guinea pigs. Physiol Behav 2014;126:8-14. doi: 10.1016/j.physbeh.2013.12.004 [published Online First: 2014/01/03]
15. Mullins PG, Rowland LM, Jung RE, et al. A novel technique to study the brain's response to pain: proton magnetic resonance spectroscopy. NeuroImage 2005;26(2):642-46. doi: 10.1016/j.neuroimage.2005.02.001 [published Online First: 2005/05/24]
16. Gussew A, Rzanny R, Erdtel M, et al. Time-resolved functional 1H MR spectroscopic detection of glutamate concentration changes in the brain during acute heat pain stimulation. NeuroImage 2010;49(2):1895-902. doi: 10.1016/j.neuroimage.2009.09.007 [published Online First: 2009/09/19]
17. Cleve M, Gussew A, Reichenbach JR. In vivo detection of acute pain-induced changes of GABA+ and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. NeuroImage 2015;105:67-75. doi: 10.1016/j.neuroimage.2014.10.042 [published Online First: 2014/12/03]
18. Mu J, Wang Q, Dun W, et al. The effects of long-term menstrual pain on pain empathy in women with primary dysmenorrhea. Pain 2021 doi: 10.1097/j.pain.0000000000002205
Figures
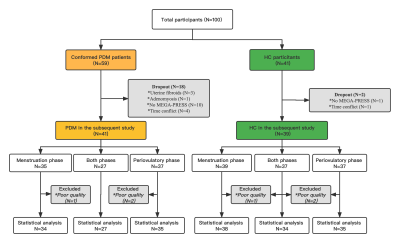
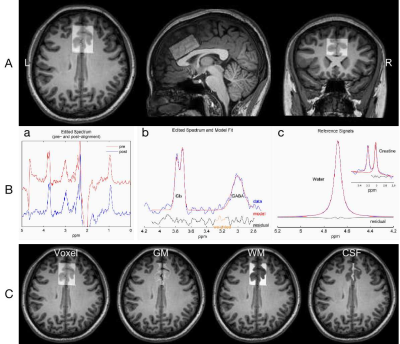
Fig. 2 Exemplary voxel placement and Gannet post-processing modules. A Localization of single voxel defined by axial/sagittal/coronal localizers in the ACC (30*40*20 mm3). B Gannet Load module shows GABA-edited difference spectrum pre (red) and post (blue) frequency/phase corrections (a). Gannet Fit module shows modelling of the Glx (at 3.74 ppm) and GABA+ (at 3.02 ppm) signals. C Gannet Segment shows segmented different tissue types in the ACC on axial T1WI in a participant (left to right: voxel location, WM, GM, CSF).
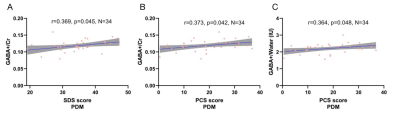
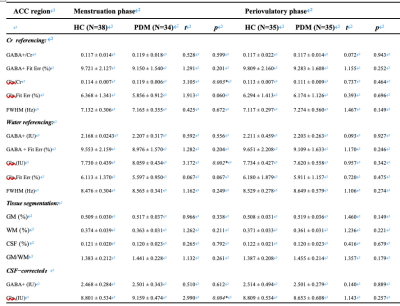
Table 1 Neurotransmitters levels and tissue compositions of PDM and HC groups.
Continuous variables are given as mean ± standard deviation; Abbreviations: HC, healthy control; PDM, primary dysmenorrhea; ACC, anterior cingulate cortex; GABA, gamma-aminobutyric acid; Glx, glutamate/glutamine compounds; Glx, glutamate/glutamine compounds; Cr, creatine; IU, institutional units; FWHM, full-width at half-maximum; Fit Err, fitting error; GM, gray matter; WM, white matter; CSF, cerebrospinal fluid.
Two simple t-test was used here.
* p < 0.05, Statistically significant
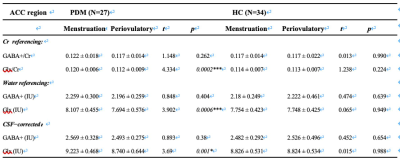
Table 2 Neurotransmitters levels compositions of PDM and HC group between menstruation and periovulatory phase.
Continuous variables are given as mean ± standard deviation; Abbreviations: PDM, primary dysmenorrhea; HC, healthy control; ACC, anterior cingulate cortex; GABA, gamma-aminobutyric acid; Glx, glutamate/glutamine compounds; Cr, creatine; IU, institutional units.
Paired t-test was used here.
* p < 0.05, Statistically significant
*** p < 0.001, Statistically significant