1703
A Novel Approach to Metabolic Imaging of Migraine and Post Traumatic Headache Using Full Brain 3D-MRSI1Neurology, Mayo Clinic, Phoenix, AZ, United States, 2Radiology, Mayo Clinic, Phoenix, AZ, United States
Synopsis
Keywords: Traumatic brain injury, Spectroscopy, migraine, 3D-MRSI, PTH
Magnetic resonance spectroscopy provides a means to quantify brain metabolites. Metabolites of interest are N-acetyl aspartate (NAA, neuronal marker), Choline (Cho, marker of cell membrane turnover), Myoinositol (Ins, astrocyte marker), Glutamate+Glutamine (Glx, neurotransmitters). Studies demonstrate metabolic changes associated with migraine such as increased Glx levels (signifying excitotoxicity), decreased NAA (suggestive of neuronal damage), as well as abnormal GABA in pain processing areas. The main goal of this work was to use using a novel, whole-brain three-dimensional magnetic resonance spectroscopy imaging (MRSI) technique to collect preliminary data on metabolic differences between migraine, post traumatic headache (PTH), and healthy controls.Introduction
Migraine is a common and debilitating neurological disorder that has a one-year prevalence of 12% in the general population (1). Approximately 39 million people in the US have migraine, which is the 2nd leading cause of years lived with disability (1). Metabolic brain alterations have been observed in people with migraine, particularly in regions relevant to migraine such as occipital cortices, thalamic nuclei, cerebellum, and cingulate (3-7) and some of them indicate modulated NAA and tCr in thalamus (3,8).Post traumatic headache (PTH) attributed to mild traumatic brain injury (mTBI) is a headache that develops within seven days of the mTBI (9). PTH is a common symptom following mTBI. In clinical settings, PTH most often has a “migraine-like” phenotype, meaning the symptoms are very similar to those of migraine (10-12).
Recent advances in 3D MRSI research sequences now allow metabolic assessment of the entire brain as compared to single-voxel metabolic assessment. The goal of this preliminary study is to test the feasibility of using 3D MRSI for investigating differences in brain metabolism between migraine, PTH, and healthy controls.
Methods
All research subjects provided written informed consent prior to participation. The migraine participants met the diagnostic criteria for migraine in accordance with ICHD-3 (9). The PTH participants met the diagnostic criteria for acute PTH attributable to mTBI in accordance with the ICHD-3 criteria (9). Healthy controls were recruited from the local community using advertisements and word-of-mouth.Acquisition: All imaging was conducted on a single 3 Tesla Siemens scanner at Mayo Clinic Arizona. The work in progress (WIP) 3D echo-planar spectroscopic imaging (3D EPSI) sequence was acquired with full brain coverage (Nx= 64, Ny=64, Nz=32) with resolution 4.375, 4.375, 6.25 mm, scan time = 17 minutes, TE /TR = 30 / 1551 on a SIEMENS 20 channel head coil. For anatomical reference, a T1-MPRAGE was acquired with 1 mm isotropic resolution (TE/TR= 3.03/2400 ms, flip angle =8 degrees).
Analysis: The MRSI, water reference, and T1-weighted images were preprocessed with MIDAS magnetic resonance spectroscopy software v2.36 (13) (http://mrir.med.miami.edu:8000/midas/) with IDL 8.8 using the standard pipeline. Quality maps were generated using MIDAS function “Quality Maps V2.0” with maximum linewidth of 13 Hz, Creatine CRLB of 40% and default parameters. Three dimensional metabolic maps for Glx, NAA, Ins, total Choline and total Creatine were assessed. MRSI maps were normalized to MNI52 space using SPM12 and smoothed with a 6 mm kernel. Only voxels with average quality (output from MIDAS) equal or greater than 2.5 were included in a two-group T-test. Group differences were compared between groups using an F-statistic. Significance was examined using a conservative p<0.05 (family wise error correction), and then again with a more uncorrected p value less than 0.001 and cluster forming threshold of 25 voxels. FSLeyes (version 0.34.2) was used to visualize results.
Results
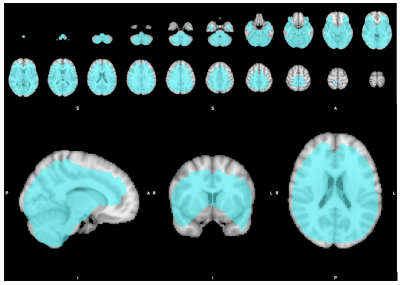
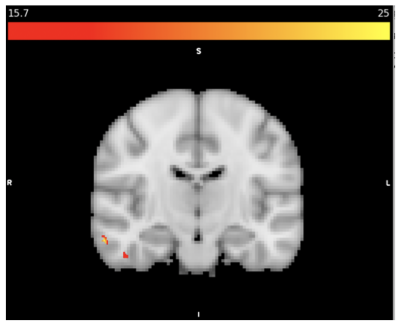
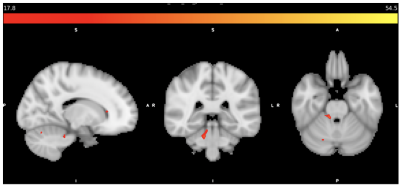
MRSI data from eleven migraine participants (10 female/1 male; mean age=34, SD=7) seven PTH participants (5 female/2 male; mean age=48, SD=18) and eight healthy controls (5 female/3 male, mean age=49, SD=14) were included. There were several metabolic differences detected between groups as shown in Table 1. The inclusion mask with average quality greater than 2.5 is shown in Figure 1. Figure 2 shows total Cr differences between HC and Migraine with F-value thresholded for p<0.001 and cluster forming threshold of 25 voxels. Figure 3 shows GLX differences between HC and PTH subjects with F-value for p<0.001 and cluster forming threshold of 25 voxels.Discussion
The right temporal region showed decreased tCr in migraine subjects relative to healthy controls (as shown in Figure 2). This finding is supported by fMRI studies that show functional changes in the temporal pole and fusiform gyrus in migraine subjects (15). Decreased Ins and tCho concentrations were found in PTH subjects compared to healthy controls. GLX was shown to be greater in the putamen, but decreased in cerebellum, fusiform and insula. Previous studies have shown lower GLX in parietal cortex (14), sensorimotor cortices (16), and cerebellum (17) of migraine subjects. Lower cerebellar GLX was previously demonstrated in migraine (17) but this is the first evidence of it in PTH. The similar migraine and PTH could have impaired cerebellar glutamatergic neurotransmission which might explain similarities in symptoms.PTH differences between HC and Migraine were apparent in the basal ganglia which is involved with pain processing. The putamen, caudate, insula, anterior cingulate, are all part of pain processing pathways (18). Differences in Ins and GLX concentrations were detected in the PTH group relative to the migraine group in the caudate, putamen, and insula. The PTH subjects showed increased GLX in putamen and decreased Ins in the insula compared to healthy controls.
The full brain 3D-MRSI did provide a novel means to detect metabolic differences in a time efficient manner. The quality map threshold masked peripheral cortical regions, some of which are known to be involved in headache and migraine. Additional technical considerations should be explored to improve full brain coverage of high quality and spatially resolved spectra.
Conclusion
Metabolic differences between migraine, PTH, and healthy controls were identified using 3D-MRSI.Acknowledgements
This study is funded by the Roubos family fund and the National Institutes of Health, National Institute of Neurological Disorders and Stroke, Award Number 1R61NS113315-01.References
1) Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 2018. 17(11): p. 954-976.
2) Fayed N, Andrés E, Viguera L, et al. Higher glutamate+glutamine and reduction of N-acetylaspartate in posterior cingulate according to age range in patients with cognitive impairment and/or pain. Acad Radiol 2014; 21: 1211-1217. 2014/07/02. DOI: 10.1016/j.acra.2014.04.009.
3) Lai TH, Fuh JL, Lirng JF, et al. Brainstem 1H-MR spectroscopy in episodic and chronic migraine. J Headache Pain 2012; 13: 645-651. 2012/10/17. DOI: 10.1007/s10194-012-0491-0.
4) Younis S, Christensen CE, Vestergaard MB, Lindberg U, Tolnai D, Paulson OB, et al. Glutamate levels and perfusion in pons during migraine attacks: A 3T MRI study using proton spectroscopy and arterial spin labeling. J Cereb Blood Flow Metab 2021;41(3):604-16.
5) Younis S, Hougaard A, Vestergaard MB, Larsson HBW, Ashina M. Migraine and magnetic resonance spectroscopy: a systematic review. Curr Opin Neurol 2017;30(3):246-62.
6) Aguila, M. R., T. Rebbeck, A. M. Leaver, J. Lagopoulos, P. C. Brennan, M. Hubscher and K. M. Refshauge (2016). "The Association Between Clinical Characteristics of Migraine and Brain GABA Levels: An Exploratory Study." J Pain 17(10): 1058-1067.
7) Sarchielli, P., R. Tarducci, O. Presciutti, G. Gobbi, G. P. Pelliccioli, G. Stipa, A. Alberti and G. Capocchi (2005). "Functional 1H-MRS findings in migraine patients with and without aura assessed interictally." Neuroimage 24(4): 1025-1031.
8) Niddam, D. M., K. L. Lai, S. Y. Tsai, Y. R. Lin, W. T. Chen, J. L. Fuh and S. J. Wang (2020). "Brain metabolites in chronic migraine patients with medication overuse headache." Cephalalgia 40(8): 851-862.
9) Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211.
10) Voormolen DC, Haagsma JA, Polinder S, Maas AIR, Steyerberg EW, Vulekovic P, et al. Post-Concussion Symptoms in Complicated vs. Uncomplicated Mild Traumatic Brain Injury Patients at Three- and Six-Months Post-Injury: Results from the CENTER-TBI Study. J Clin Med 2019;8(11).
11) Ashina H, Iljazi A, Al-Khazali HM, Ashina S, Jensen RH, Amin FM, et al. Persistent post-traumatic headache attributed to mild traumatic brain injury: Deep phenotyping and treatment patterns. Cephalalgia 2020;40(6):554-64.
12) Howard L, Dumkrieger G, Chong CD, Ross K, Berisha V, Schwedt TJ. Symptoms of Autonomic Dysfunction Among Those with Persistent Posttraumatic Headache Attributed to Mild Traumatic Brain Injury: A Comparison to Migraine and Healthy Controls. Headache 2018;58(9):1397-407.
13) Ebel, A., Maudsley, A. Detection and Correction of Frequency Instabilities for Volumetric 1H Echo-Planar Spectroscopic Imaging, MRM 53:465-469 (2005)
14) Paucar, M., T. Granberg, K. Lagerstedt-Robinson, E. Waldenlind, S. Petersson, L. Nordin and P. Svenningsson (2020). "SLC1A3 variant associated with hemiplegic migraine and acetazolamide-responsive MRS changes." Neurol Genet 6(4): e474.
15) Schwedt, T. J., B. L. Schlaggar, S. Mar, T. Nolan, R. S. Coalson, B. Nardos, T. Benzinger and L. J. Larson-Prior (2013). "Atypical resting-state functional connectivity of affective pain regions in chronic migraine." Headache 53(5): 737-751.
16) Bell, T., M. Stokoe, A. Khaira, M. Webb, M. Noel, F. Amoozegar and A. D. Harris (2021). "GABA and glutamate in pediatric migraine." Pain 162(1): 300-308.
17) Dichgans, M., J. Herzog, T. Freilinger, M. Wilke and D. P. Auer (2005). "1H-MRS alterations in the cerebellum of patients with familial hemiplegic migraine type 1." Neurology 64(4): 608-613.
18) Valfre, W., I. Rainero, M. Bergui and L. Pinessi (2008). "Voxel-based morphometry reveals gray matter abnormalities in migraine." Headache 48(1): 109-117.
Figures