1692
Incorporation of View-Sharing and KWIC Filtering into XD-GRASP Reconstruction Improves Spatial Resolution in Thoracic NC-MRA1Department of Biomedical Engineering, Northwestern University, Evanston, IL, United States, 2Department of Radiology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 3Department of Pediatric, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 4Division of Cardiology, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States, 5Department of Medical Imaging, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States
Synopsis
Keywords: Vessels, Image Reconstruction
Conventional thoracic non-contrast magnetic resonance angiography (NC-MRA) often produces suboptimal image quality in pediatric patients with congenital heart disease (CHD) and results in lengthy scan times. We recently developed a NC-MRA pulse sequence using a stack-of-stars k-space sampling pattern with XD-GRASP reconstruction and evaluated its performance in adults. In this study, we sought to improve our NC-MRA pulse sequence by incorporating view-sharing (VS) and k-space weighted image contrast (KWIC) filtering to reduce blurring, which is critical for pediatric patients who have smaller hearts and faster heart rates than adults. We evaluated it performance against contrast-enhanced MRA in pediatric patients.
Introduction
Thoracic vascular anatomy assessment is essential to patients with congenital heart disease (CHD)1. Contrast enhanced MR angiography (CE-MRA) detects vascular stenosis, dilation and other abnormalities2. However, CE-MRA relies on gadolinium-based contrast agent (GBCA) which adds risk and cost and could lead to tissue deposition of gadolinium for those children who will require lifetime follow-up3. A non-contrast approach is T2-prepared NC-MRA with b-SSFP readout4; however, conventional NC-MRA often result in long scan times that are unpredictable. We have recently developed a self-navigated NC-MRA pulse sequence using stack-of-stars k-space sampling pattern and XD-GRASP reconstruction5 and validated its performance in adults6. Our initial experience with our NC-MRA in children produced suboptimal results, because children often have smaller hearts and faster heart rates. In this study, we sought to improve our NC-MRA by incorporating view-sharing (VS) and k-space weighted image contrast (KWIC) filtering7 to reduce spatial blurring from over regularization. Pediatric CHD patients were enrolled to evaluate performance with respect to mage quality and aortic diameters, where CE-MRA was used as reference.Methods
Human Subjects & Pulse Sequence: We retrospectively identified 14 pediatric patients with CHD (mean age = 9±2 years, 11/3 males/females) who underwent a clinical standard CE-MRA and our proposed NC-MRA6. The relevant imaging parameters for clinical CE-MRA and our proposed NC-MRA are shown in Table 1. Both CE-MRA and NC-MRA were acquired in a coronal plane.Image reconstruction: Respiratory motion tracking is an inevitable pre-processing step for free-breathing MRA. Based on a previous work6, we evenly rebinned the acquired signal shots into six respiratory phases, as shown in Figure 1a and Figure 1b for default XD-GRASP reconstruction. We used total variation along the respiratory dimension as the sparsifying transform. We used VS to add more samples at the periphery of k-space, where in turn will increase spatial resolution, and also added KWIC filtering to exclude the center of k-space in VS k-space lines (see Figure 1c), which is necessary to minimize motion blurring. We empirically determined that view sharing k-space lines from two adjacent respiratory states achieved a good balance between improvement of spatial resolution and reduction in motion blurring.
Image quality analysis: For quantitative evaluation, we calculated the blur metric8 and measured two orthogonal aortic diameters (longest and its orthogonal) at seven standardized locations established by guidelines9 (2: sinotubular junction; 3: mid ascending aorta; 4: proximal aortic arch; 5: mid aortic arch; 6: proximal descending thoracic aorta; 7: mid descending aorta; 8: aorta at diaphragm). For blur metric calculation, we limited the analysis to the heart region by manually cropping the FOV. Aortic diameters were drawn using the 3D MPR tool in Circle CVI42. We tested for parameter normality using the Shapiro-Wilk test. We compared the blur metric calculated from the NC-MRA with XD-GRASP alone, and XD-GRASP with VS and KWIC filtering using two-tailed, paired t-test (Wilcoxon signed-rank, if not normally distributed). We compared the two orthogonal aortic diameters measured at seven standardized locations individually between clinical CE-MRA and our proposed NC-MRA - the group with the smallest blur metric - using two-tailed, paired t-test (Wilcoxon signed-rank, if not normally distributed). In addition, linear-regression and Bland-Altman analyses were conducted on vessel diameters to determine the coefficient of determination (R2) and levels of agreement. A p < 0.05 was considered statistically significant for each statistical test.
Results
According to the Shapiro-Wilk test, the blur metric and two orthogonal aortic diameters at all 7 locations were normally distributed (statistic: [0.08, 0.97]), with the exception of vessel diameters at location 3 (p < 0.05). Figure 2 shows representative MRA images: clinical CE-MRA (first row), NC-MRA with XD-GRASP alone (second row), and XD-GRASP with VS and KWIC filtering (third row). Among NC-MRA reconstructions, incorporating VS+KWIC produced better image quality as shown. As summarized in Table 2, VS with KWIC filtering significantly (p<0.05) reduced the blur metric (0.35±0.03) compared with default XD-GRASP (0.40±0.04). Thus, we used XD-GRASP with VS+KWIC for aortic diameters comparison with CE-MRA. There were no significant differences in the two orthogonal vessel diameters at all 7 locations (p>0.05), except for the longest diameter at mid ascending aorta (p<0.05). Figure 3 shows the scatter plots of linear regression and Bland-Altman analyses where vessel diameters measured on CE-MRA and NC-MRA with VS+ KWIC are strongly correlated (R2 = 0.87) and in good agreement (absolute mean difference ≤ 0.2 mm and 95% confidence interval ≤ 6.9 mm).Conclusion
Incorporating VS + KIWC into XD-GRASP reconstruction improved spatial resolution for our accelerated, free-breathing NC-MRA images, which resulted in relatively accurate aortic diameters in pediatric patients with CHD. Future study includes head-to-head comparison with conventional NC-MRA in pediatric patients with CHD.Acknowledgements
This work is supported by National Institutes of Health (R01HL116895, R21AG055954, R01HL151079, R21EB030806A1) and American Heart Association (19IPLOI34760317, 949899, 903375).
References
1. Ntsinjana HN, Hughes ML and Taylor AM. The role of cardiovascular magnetic resonance in pediatric congenital heart disease. J Cardiovasc Magn Reson. 2011;13:51.
2. Naehle CP, Kaestner M, Muller A, Willinek WW, Gieseke J, Schild HH and Thomas D. First-pass and steady-state MR angiography of thoracic vasculature in children and adolescents. JACC Cardiovasc Imaging. 2010;3:504-13.
3. McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE and Eckel LJ. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology. 2015;275:772-82.
4. Srichai MB, Kim S, Axel L, Babb J and Hecht EM. Non-gadolinium-enhanced 3-dimensional magnetic resonance angiography for the evaluation of thoracic aortic disease: a preliminary experience. Tex Heart Inst J. 2010;37:58-65.
5. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK and Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72:707-17.
6. Haji-Valizadeh H, Collins JD, Aouad PJ, Serhal AM, Lindley MD, Pang J, Naresh NK, Carr JC and Kim D. Accelerated, free-breathing, noncontrast, electrocardiograph-triggered, thoracic MR angiography with stack-of-stars k-space sampling and GRASP reconstruction. Magn Reson Med. 2019;81:524-532.
7. Song HK and Dougherty L. k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med. 2000;44:825-32.
8. Crete F DT, Ladret P, Nicolas M. The blur effect: perception and estimation with a new no-reference perceptual blur metric. SPIE. 2007.
9. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr., Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG and Williams DM. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology,American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27-e129.
Figures

Table 1 Summary of relevant pulse sequence parameters.
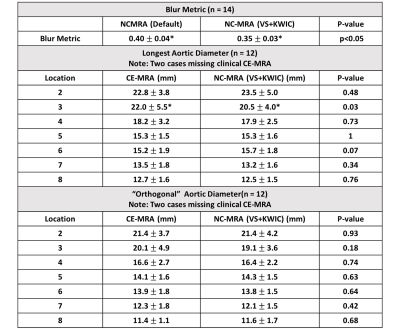
Table 2 Summary of the blur metrics and two aortic diameters (longest and its orthogonal) at seven standardized locations. Values are presented as means ± standard deviations. Location 2: sinotubular junction; Location 3: mid ascending aorta; Location 4: proximal aortic arch; Location 5: mid aortic arch; Location 6: proximal descending thoracic aorta; Location 7: mid descending aorta; Location 8: aorta at diaphragm. *P < 0.05 was considered statistically significant.

Figure 1 A schematic describing how view-sharing (VS) and k-space weighted image contrast (KWIC) filtering are incorporated into XD-GRASP framework along the respiratory state dimension.
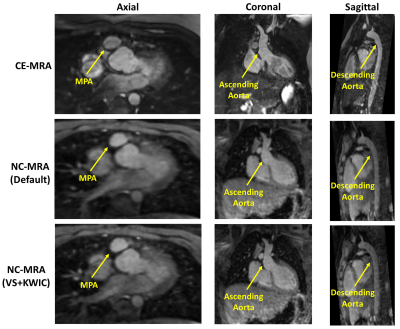
Figure 2 Representative images from one patient. From left to right: axial plane, coronal plane and sagittal plane. From first row to third row: CE-MRA, NC-MRA with default XD-GRASP alone, XD-GRASP with VS+KWIC.
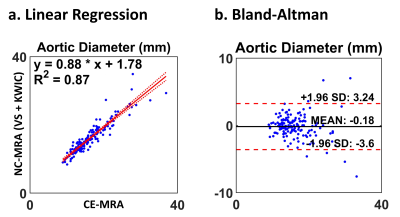
Figure 3 (a) Linear regression and (b) Bland-Altman plots of aortic diameters comparing CE-MRA and NC-MRA with VS and KWIC filtering. The plots include two orthogonal aortic dimensions at all seven locations.