1678
The Value of Pelvic Skeletal Muscles to Identify the Low-Risk Rectal Cancers with Poor Prognosis1The First Hospital of Jilin University, Changchun, China
Synopsis
Keywords: Cancer, Muscle
The low-risk rectal cancer (RC) has no need for postoperative treatment after total mesorectal excision (TME). However, there are some low-risk RC patients without postoperative-treatments had subsequent metastasis and recurrence. Skeletal muscles are gaining more attention which shown to be associated with morbidity and mortality in caners. Therefore, this study established a radiomics model based on pelvic skeletal muscles on MRI to identify the low-risk rectal cancer (RC) with poor prognosis. Our research shows that the novel radiomic signatures could be used to predict disease-free survival (DFS) in low-risk RC to help clinicians improve the treatment decision making followed TME.Introduction
The low-risk rectal cancer (RC) has no need for postoperative treatment after total mesorectal excision (TME), which is defined as pT1–pT3a/b, N0, MRF clear, lymphovascular invasion negative (LVI-) and perineural invasion negative (PNI-) by the postoperative histopathology.1 However, there are some low-risk RC patients without postoperative-treatments had subsequent metastasis and recurrence. Therefore, how to identify the patients of low-risk RC with poor prognosis is very important to make the follow-up treatment plan after TME. Recently, as part of body composition, skeletal muscles are gaining more attention which shown to be associated with morbidity and mortality in caners and following abdominal surgery.2,3 MRI is recommended for RC staging, and pelvic muscles can be clearly shown1,4, however, it is difficult for radiologists to find potential quantitative characteristics of muscles in images. Currently, radiomics5 may improve the accuracy of diagnosis, prognosis and prediction by extracting and analyzing the first-order and high-order image features of medical images. Therefore, the purpose of this study was to build a radiomics model using skeletal muscles based on MRI to predict disease-free survival (DFS) , so as to differentiate the underlying poor outcome patient in low-risk RC.Methods
Study Population: A total of 99 postoperative histopathological proved RC (allocated to a training and testing set with a 7:3 ratio) with low risk factors (cT1–cT3a/b, N0, MRF clear, no EMVI, no LVI and PNI) were recruited in our study. All patients underwent MR examination preoperatively, had no postoperative-treatments followed by TME, and followed up 2-6 years. Our institutional review board approved this retrospective study and waived the requirement for informed consent.MRI Acquisition: MRI examinations were performed with a 3.0-T system (uMR780; Shanghai United Imaging Healthcare Co., Ltd.) with phased-array surface coils. 2D fast spin-echo (FSE) T2WI was performed in coronal planes, repetition time 4900 ms, echo time 153.72 ms, slice thickness 4 mm, gap 0.4 mm, field of view 320x320 mm2, refocus flip angle 90.
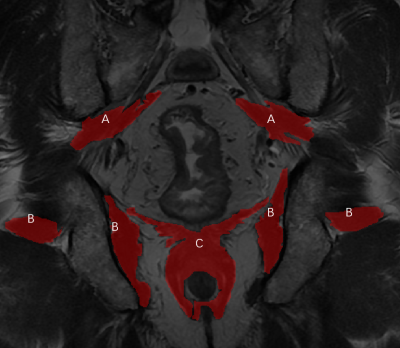
Annotation: Two gastrointestinal radiologists (G.J. and F.Y., with 5 and 11 years of experience in rectal MRI, respectively) annotated the area of interests (ROIs) together. When opinions were consistent, the label would be output. ITK-SNAP software (version 3.6, www.itk-snap. org) was used for manual segmentation of 2D MR images, and the muscles were delineated layer by layer on the coronal T2WI sequence, including bilateral piriformis, obturator internus and perianal complex. (Figure.1)
Statistical Analysis: Statistical Analysis: Total 33 radiomic signatures based on 1257 3D features was generated using the least absolute shrinkage and selection operator (LASSO) Cox regression model by 5 folds cross validation. Then 8 radiomic signatures with p value less than 0.1 were selected by Cox single factor. Finally, 3 radiomic signatures with p value less than 0.05 were selected by Cox multi factor analysis. (Figure.2) The Cox-score is obtained by the coefficient of the Cox model and the value of radiomic signatures. The Cox-score with DFS was investigated by Kaplan-Meier survival curves. Survival curves were compared by the log-rank test. One model was built and assessed for their predictive values, using the Harrell concordance index.
Results
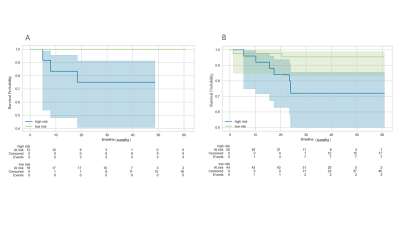
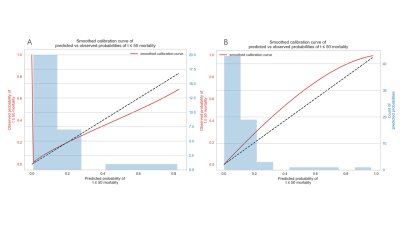
The Cox-score stratified patients into low- and high-risk groups for DFS in the training set (P = 0.006), and was successfully validated in the testing set (P =0.031). The model with 3 radiomic signatures had good performance in training set (C index=0.776, 95% confidence interval [CI] 0.598-0.914) and testing set (C index=0.753, 95% confidence interval [CI] 0.591-0.939). (Figure.3) The calibration curves depicted consistency between the predicted and observed outcomes. (Figure.4)Discussion
In agreement with existing publications based on muscles,6 we successfully established a radiomics model using pelvic muscles to predict DFS in low-risk RC, which demonstrated that the model could be used to differentiated the poor prognosis patients. The above objectively results would help clinicians to screen the bad outcome patients and ultimately improve the treatment decision making followed TME. Muscle is the distinct entity from other markers of physiological reserve, which is a considered marker of overall health. And in our study, we found 3 radiomic signatures of pelvic muscles associated with poor prognosis, which maybe new biomarkers to make a novel risk stratification for RC. However, our data is biased, which is consistent with clinical reality. Further, we will increase the sample size, hoping to build a more robust and generalized model.Conclusion
The novel radiomic signatures could be used to predict DFS in patients with low-risk RC. The radiomic model has the ability to estimate DFS (P=0.006, 0.031 in training set and in validation set, respectively), and may help guide individualized treatment in such patients.Acknowledgements
No acknowledgements found.References
1. Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology 2017;28(suppl_4):iv22-iv40.
2. Lee CM, Kang J. Prognostic impact of myosteatosis in patients with colorectal cancer: a systematic review and meta-analysis. Journal of Cachexia Sarcopenia and Muscle 2020;11(5):1270-1282. 3. Weerink LBM, van der Hoorn A, van Leeuwen BL, de Bock GH. Low skeletal muscle mass and postoperative morbidity in surgical oncology: a systematic review and meta-analysis. Journal of Cachexia Sarcopenia and Muscle 2020;11(3):636-649.
4. Benson AB, Venook AP, Al-Hawary MM, et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw 2020;18(7):806-815.
5. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, Aerts HJWL. Radiomics: Extracting more information from medical images using advanced feature analysis. European Journal of Cancer 2012;43(4):441-446.
6. Body S, Ligthart MAP, Rahman S, et al. Sarcopenia and Myosteatosis Predict Adverse Outcomes After Emergency Laparotomy A Multi-center Observational Cohort Study. Annals of Surgery 2022;275(6):1103-1111.
Figures