1675
Continuous-time random walk diffusion model combined with VI-RADS to predict muscle invasion of bladder cancer1Department of Radiology, Peking University First Hospital, Beijing, China, 2MR Collaboration, Central Research Institute, Shanghai United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: Cancer, Bladder, diffusion/other diffusion techniques
Accurately differentiation of MIBC from non-muscle-invasive bladder cancer (NMIBC) is essential for selecting the optimal treatment plan and obtaining a better prognosis for BCa patients. In this study, the role of the CTRW model, VI-RADS and their combination in characterizing MIBC was evaluated. The CTRW parameters combined with VI-RADS could provide significantly better diagnostic performance for MIBC determination than the VI-RADS score alone. The CTRW model could serve as a compliment to VI-RADS and provide added value for predicting muscle invasion of bladder cancer.Introduction
Bladder cancer (BCa) is one of the most common malignant tumors of the urinary system with high morbidity and mortality1, among which about 15%- 30% are muscle-invasive bladder cancer (MIBC)2. Accurately differentiation of MIBC from non-muscle-invasive bladder cancer (NMIBC) is essential for selecting the optimal treatment plan and obtaining a better prognosis for BCa patients. The Vesical Imaging Reporting and Data System (VI-RADS) based on multi-parametric magnetic resonance imaging (mp-MRI) has demonstrated good performance in distinguishing NMIBC from MIBC3,4. Among mp-MRI, apparent diffusion coefficient (ADC) derived from diffusion-weighted imaging (DWI) plays a very important role in VI-RADS by quantifying the diffusivity of water molecules within tissues. Considering the varying structural complexity in cancer, a non-gaussian diffusion model, continuous-time random-walk (CTRW) model5 may provide a more comprehensive characterization for BCa. Thus the aim of our study is to evaluate the ability of high b-value DWI with the CTRW diffusion model combined with the vesical imaging-reporting and data system (VI-RADS) for muscle-invasive diagnosis of bladder cancer.Methods
A total of 55 patients (13 female; 25 with muscle layer invasion) with pathologically confirmed bladder cancer were prospectively enrolled. All patients underwent the bladder MRI on a 3.0 T MRI scanner (uMR790, United Imaging Healthcare, Shanghai, China). DWI was performed using 11 b-values (0, 50, 100, 200, 400, 800, 1000, 1500, 2000, 2500, 3000 s/mm2). Two radiologists evaluated the VI-RADS score based on T2WI and DWI and outlined the tumor on the slice with the maximum size, excluding necrotic areas. The multi-b-value DWI was fitted voxel-by-voxel based on the CTRW model equation5,6 using an Levenberg–Marquardt non-linear fitting method in MATLAB 2021a (MathWorks, Natick, MA, USA). Then three CTRW model parameters, including an anomalous diffusion coefficient Dm, and two parameters related to temporal and spatial diffusion heterogeneity α and β, respectively6, were obtained. The Dm, α, and β were compared between NMIBC and MIBC using Independent student’s t-test or Mann-Whitney U test according to their normality. The CTRW parameters and VI-RADS were combined by the binary logistic regression analysis. The diagnostic performance of single CTRW parameter, VI-RADS and their combination in characterizing MIBC were evaluated by receiver operating characteristic (ROC) analysis. Statistical significance was considered when P < 0.05.Results
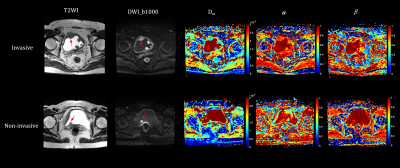
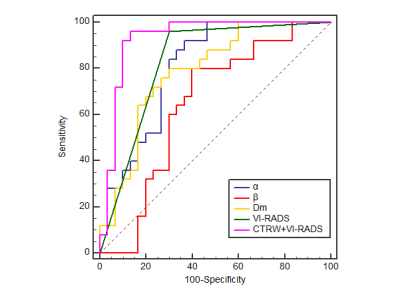
Representative images of NMIBC and MIBC are shown in Figure 1. The Dm and α values were significantly lower for MIBC than NMIBC (Dm: 1.01 ± 0.24 μm2/ms vs. 1.43±0.56 μm2/ms; α: 0.69±0.13 vs. 0.82±0.10; all p<0.001). β was not statistically different between MIBC and NMIBC (p=0.07). The area under the curve (AUC) of Dm, α, and VI-RADS for MIBC determination was 0.79, 0.81, and 0.83, respectively (Figure 2). The combined diffusion parameters and VI-RADS score further improved the performance for MIBC diagnosis (AUC=0.93), which was significantly better than the VI-RADS score (p=0.02), D (p=0.01), α (p=0.01) and β (p<0.001) alone.Discussion
In this study, the role of the CTRW model, VI-RADS and their combination in characterizing MIBC was evaluated. Importantly, incorporation of CTRW diffusion model into VI-RADS significantly improved the diagnostic performance of VI-RADS alone, suggesting that CTRW could yield the additional useful information in determining muscle invasion status of BCa. Lower Dm values in MIBC compared with NMIBC were similar to the previous study, which may be related to the increased cellularity and thus decreased extracellular space tortuosity in MIBC7,8. The parameters α describes the variable time of water molecules to make a move, thus it reflects the degree of non-Gaussian diffusion behavior in temporal6. As the degree of temporal diffusion heterogeneity increases, α decreases accordingly6. In our study, Lower α values in MIBC were found, which indicate that the water molecule was more likely to be “trapped” due to the more complex and heterogeneous structure of MIBC in temporal dimension.Conclusion
The CTRW parameters combined with VI-RADS could provide better diagnostic performance for MIBC determination than the VI-RADS score alone. The CTRW model could serve as a compliment to VI-RADS and provide added value for predicting muscle invasion of bladder cancer.Acknowledgements
No acknowledgementsReferences
1. Antoni, Sebastien et al. “Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends.” European urology vol. 71,1 (2017): 96-108. doi:10.1016/j.eururo.2016.06.010
2. Gregg, Justin R et al. “Guideline-based management of non-muscle invasive bladder cancer.” Indian journal of urology : IJU : journal of the Urological Society of India vol. 31,4 (2015): 320-6. doi:10.4103/0970-1591.163305
3. Del Giudice, Francesco et al. “Systematic Review and Meta-Analysis of Vesical Imaging-Reporting and Data System (VI-RADS) Inter-Observer Reliability: An Added Value for Muscle Invasive Bladder Cancer Detection.” Cancers vol. 12,10 2994. 15 Oct. 2020, doi:10.3390/cancers12102994
4. Zhang, Nieke et al. “Diagnostic Accuracy of Multi-Parametric Magnetic Resonance Imaging for Tumor Staging of Bladder Cancer: Meta-Analysis.” Frontiers in oncology vol. 9 981. 4 Oct. 2019, doi:10.3389/fonc.2019.00981
5. Ingo, Carson et al. “On random walks and entropy in diffusion-weighted magnetic resonance imaging studies of neural tissue.” Magnetic resonance in medicine vol. 71,2 (2014): 617-27. doi:10.1002/mrm.2470 6. Karaman, M Muge et al. “Differentiating low- and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values.” Magnetic resonance in medicine vol. 76,4 (2016): 1149-57. doi:10.1002/mrm.26012
7. Takeuchi, Mitsuru et al. “Urinary bladder cancer: diffusion-weighted MR imaging--accuracy for diagnosing T stage and estimating histologic grade.” Radiology vol. 251,1 (2009): 112-21. doi:10.1148/radiol.2511080873
8. Kobayashi, Shuichiro et al. “Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness.” European radiology vol. 21,10 (2011): 2178-86. doi:10.1007/s00330-011-2174-7