1660
Self-supervised Denoising of Pulmonary Perfusion Imaging in Human Subjects and Swine1Biomedical, Biological and Chemical Engineering, University of Missouri Columbia, Columbia, MO, United States, 2Radiology, University of Missouri Columbia, Columbia, MO, United States, 3Biomedical Sciences, University of Missouri Columbia, Columbia, MO, United States
Synopsis
Keywords: Lung, Machine Learning/Artificial Intelligence, Self-supervised Denoising
Self-supervised learning denoising networks can be applied to noisy only datasets when the clean-noisy pairs are not available, which is suitable for dynamic contrast-enhanced (DCE) pulmonary imaging where SNR is low and no ground truth clean image can be acquired. Blind-spot network with asymmetric pixel-shuffle downsampling (AP-BSN) was trained to utilize the advantages of self-supervised BSN and improve the denoising performance for pixel-wise independent and dependent noise. AP-BSN denoised images showed improved image quality by human reader assessment. AP-BSN showed generalization ability from human subjects to swine and from 1.5T to 3T.Introduction
If high quality images could be obtained, contrast-enhanced pulmonary perfusion MRI could be useful for the assessment of pulmonary arterial diseases. The challenges are low proton density and short T2* of lung, which lead to low signal-to-noise ratio (SNR) [1]. Supervised deep learning (DL) has already demonstrated state-of-the-art performance for denoising images and has been introduced into MRI [2]. However, acquiring a large number of clean-noisy pairs is not possible for dynamic contrast-enhanced (DCE) pulmonary imaging. Recently, self-supervised denoising using Blind-Spot Network (BSN) has gained attention and been validated to achieve comparable performance with supervised denoising in natural images [3]. Most of the denoising filter and DL networks assume pixel-wise independent noise. In MRI, however, the assumption is not met due to the spatially varying noise caused by intensity inhomogeneity, parallel imaging, or surface coil-based acquisition [4]. The noise in pulmonary perfusion MRI was especially spatially dependent because of the strong difference between low-intensity lung tissues and high-intensity other tissues [5]. Pixel-shuffle downsampling (PD) [6] is a novel concept to break down the spatial correlation in real-world noise. Since noise signals are correlated with the neighboring pixels, downsampling may break the dependency. In this work, we propose to use self-supervised denoising for pulmonary perfusion MRI via AP-BSN [3], which is the combination of BSN [7] and asymmetric PD [3]. The purpose of this study was to assess the potential utility of self-supervised denoising algorithm on pulmonary perfusion imaging, perform a human reader assessment of image quality, and assess the generalization ability of the model (human vs swine and 1.5T vs 3T).Methods
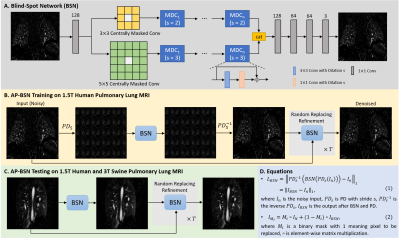
Datasets: Contrast-enhanced pulmonary perfusion images were acquired with 3D TWIST sequence on 1.5T Aera scanner for 27 human datasets with suspected pulmonary embolism, and 3T Vida scanner (Siemens Healthcare, Erlangen, Germany) for one swine with cardiac infarction. Image parameters were: in-plane spatial resolution of 0.94-1.64mm2, slice thickness=1.2-5mm, matrix size=256-352×112-246, number of slices=35-175, TE=0.79-1.02ms, TR=1.76-2.73ms and flip angle=11-30°. 20 human 3D datasets with 2504 images were used for training; the other 7 human datasets with 1032 images and one swine data with 114 images were used for testing.Network: BSN is one of the representative self-supervised networks to reconstruct a clean pixel from the surrounding noisy pixels without referring the exact input pixel (Fig. 1A). However, due to the spatially correlated MRI noise, the performance of BSN alone will be suboptimal. By combining PD into BSN, which break down the spatial correlation of the noise, BSN could be generalized to real acquired MRI data. By adopting a bigger downsampling stride in training (Fig. 1B) and smaller stride in testing (Fig. 1C), the asymmetric strategies would reach a good balance of keeping sharpness and removing bigger artifacts such as motion and aliasing artifacts. Loss function was shown in Fig. 1D equation (1). Random-replacing refinement were further used to suppress artifacts from PD and keep sharpness. T randomized binary masks were generated to combine the original noisy image and the denoised intermediate as shown in Fig. 1D equation (2). For pulmonary perfusion MRI, the following hyperparameter settings achieved the best performance: AP5/1 with stride=5 in training and stride=1 in testing and T=16. 3×3 and 5×5 centrally masked convolutions were used as two paths in BSN, with each path containing 9 dilated convolutional blocks (Fig. 1A). The supervised learning DnCNN [8] trained by natural images was used for comparison with the proposed method.
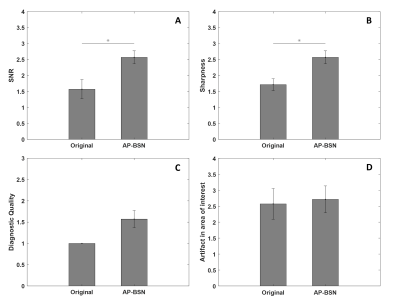
Assessment: Due to the lack of ground truth, image quality was assessed by a radiologist (>20 years experience with MR lung imaging) by an overall consideration of SNR, sharpness, diagnostic utility and artifacts in area of interest. Images were scored from 1 to 5, with a higher score associated with better quality.
Results
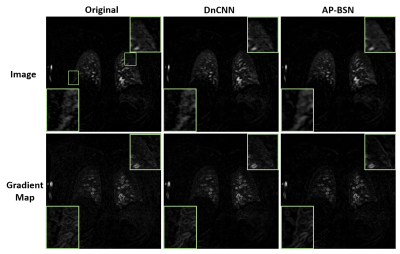
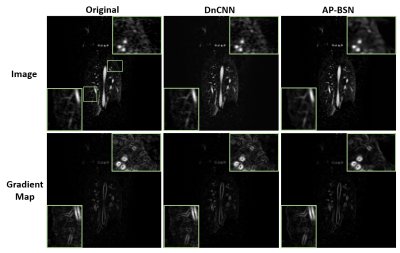
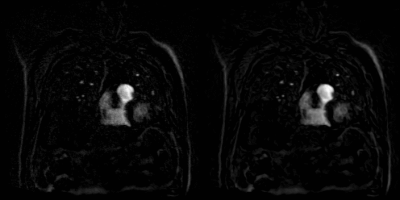
Fig. 2 shows examples comparing the original noisy image, DnCNN and AP-BSN results for a patient with acute nonocclusive pulmonary emboli on a 1.5T scanner. Gradient maps are shown to compare the sharpness by applying an image gradient filter. Fig. 3 shows an infarcted swine example acquired on a 3T scanner. In both examples, DnCNN and AP-BSN showed less noise than original image, but AP-BSN shows the cleanest image. AP-BSN also maintains the sharpness, where the edges of images are clearly shown in the image gradient maps. Fig. 4 (movie) shows the comparison of 3D original images and AP-BSN denoised images, where AP-BSN achieved better image quality than the original images with a good balance of denoising and sharpness maintaining. Figure 5 provides the quantitative image quality comparison by scoring from the radiologist, which showed improved image quality after AP-BSN denoising.Discussion and Conclusions
This self-supervised denoising network utilized self-supervised blink-spot network and asymmetric PD to resolve spatially correlated MRI noise and with post-processing random replacing refinement to further improve the performance. AP-BSN on perfusion lung MRI images shows promising denoising performance on both human and swine testing datasets. This network has the generalization ability from human subjects to large animals and to different field strengths, and has the potentials to be generalized to other MRI data where the acquisition of training targets is not easy or impossible.Acknowledgements
No acknowledgement found.References
[1] Ley S, Ley-Zaporozhan J. Pulmonary perfusion imaging using MRI: clinical application. Insights Imaging 2012;3(1):61-71.
[2] Lopez MM, Frederick JM, Ventura J. Evaluation of MRI Denoising Methods Using Unsupervised Learning. Front Artif Intell 2021;4.
[3] Lee, Wooseok, Sanghyun Son, and Kyoung Mu Lee. "AP-BSN: Self-Supervised Denoising for Real-World Images via Asymmetric PD and Blind-Spot Network." Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition. 2022.
[4] Manjón, José V., et al. "Adaptive non‐local means denoising of MR images with spatially varying noise levels." Journal of Magnetic Resonance Imaging 31.1 (2010): 192-203.
[5] Wild, Jim M., et al. "MRI of the lung (1/3): methods." Insights into imaging 3.4 (2012): 345-353.
[6] Zhou, Yuqian, et al. "When awgn-based denoiser meets real noises." Proceedings of the AAAI Conference on Artificial Intelligence. Vol. 34. No. 07. 2020.
[7] Krull, Alexander, Tim-Oliver Buchholz, and Florian Jug. "Noise2void-learning denoising from single noisy images." Proceedings of the IEEE/CVF conference on computer vision and pattern recognition. 2019.
[8] Zhang, Kai, et al. "Beyond a gaussian denoiser: Residual learning of deep cnn for image denoising." IEEE transactions on image processing 26.7 (2017): 3142-3155.
Figures