1658
Development and validation of combined Ki67 status prediction model for intrahepatic cholangiocarcinoma based on MRI radiomics
Xianling Qian1, Changwu Zhou1, Yunfei Zhang2, Yongming Dai2, and Mengsu Zeng1
1Zhongshan Hospital, Fudan University, Shanghai, China, 2MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
1Zhongshan Hospital, Fudan University, Shanghai, China, 2MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: Liver, Radiomics
In the past decades, the incidence and mortality of intrahepatic cholangiocarcinoma (ICC) have been on the rise. A nuclear antigen named Ki67 is a poor prognostic predictor and an attractive therapeutic target in patients with ICC. Therefore, accurate prediction of Ki67 status in ICC patients is a predictor for treatment efficacy evaluation and outcome prediction. In this study, we established a multiparametric model for predicting Ki67 status in ICC patients preoperatively.Introduction
ICC, as one type of primary liver cancer (PLC), accounts for 10%-15% of PLC, with increasing incidence and mortality over the past few decades (1–3). Ki67 protein is a nuclear antigen related to cell proliferation and positively correlated with advanced ICC (4, 5). Recently, Ki67 molecular targeted therapies are expected to improve the prognosis of ICC patients (6). Therefore, accurate prediction of Ki67 status in ICC patients is a predictor for treatment efficacy evaluation and outcome prediction, while it is difficult to determine Ki67 status of ICC lesions by routine imaging and laboratory tests. Currently, radiomics is defined as a high-throughput extraction of numerous image features from medical images, and independent features are applied to the construction of diagnostic, predictive and prognostic models (7). However, the development and validation of Ki67 status prediction model for ICC lesions based on radiomics features has not yet been studied. Thereby, we combined Ki67 prediction model incorporates clinicoradiological and radiomics features for predicting Ki67 status in ICC patients preoperatively.Methods
Data Acquisition: Preoperative contrast-enhanced MR images were collected from 178 patients with postoperative pathologically confirmed ICC, and a time-independent test cohort of 60 ICC patients was used for validation. The demographic data, preoperative serum AFP, carcinoembryonic antigen (CEA), CA199, history of hepatitis B virus (HBV) serum markers and HBV-DNA loads were retrospectively retrieved. All PLC samples were sampled using 7-point baseline sampling protocol. Histopathological features including lesion number, Edmondson-Steiner grade and Ki67 status were evaluated by two experienced abdominal pathologists. Anti-human Ki67 rabbit monoclonal antibodies were used with a dilution of 1:50 in immunohistochemistry, and the Ki67 labeling index (LI) was recorded. Contrast-enhanced MRI was performed by intravenous injection of 0.2 mmol/kg Gd-DTPA, followed immediately by a 20 ml saline flush at 2 ml/s. Imaging features were assessed independently by 2 blinded and experienced abdominal radiologists. The tumor segmentation was performed in the ITK-SNAP software and the extraction of radiomics features was performed by the uAI Research Portal (Version: 20210730), in which PyRadiomics was embedded. After feature selections, we constructed clinical model and imaging model based on corresponding independent clinicoradiological predictors. Independent clinicoradiological predictors of Ki67 status were determined by multivariate analysis. Optimal radiomics features were selected byLASSO logistic regression and linear discriminant analysis was used to construct combined models. The prediction efficacy of combined model was assessed by ROC curve, and verified by its calibration, decision and clinical impact curves.Results
The study flowchart of the enrolled patients and radiomics analysis were exhibited in Figure 1 and Figure 2. The clinical+imaging model shows an unsatisfactory predictive efficiency (AUCtraining=0.714, AUCvalidation=0.535) and the AUCs of 6 single MR sequence models constructed with robust radiomics features are displayed (Figure 3A-B). The final three-sequence radiomics model incorporates T1, T1V and T1D models shows a satisfying predictive performance (AUCtraining=0.899, AUCvalidation=0.837) (Figure 3D-E). The combined Ki67 prediction model incorporating clinical, imaging and radiomics model achieves excellent predictive efficiency in training (AUC=0.930, 95%CI: 0.886-0.974), validation (AUC=0.870, 95%CI: 0.775-0.965) and test (AUC=0.804, 95%CI: 0.699-0.910) cohorts (Figure 3C-E). The flowchart of the combined Ki67 prediction model is shown in Figure 4. Calibration curves show the goodness of fit between the predicted Ki67 status by using the combined Ki67 prediction model and actual Ki67 status in the training (p=0.781) and validation (p=0.732) cohorts (Figure 5A-B). Decision curves show that radiomics model, imaging+radiomics model and the combined Ki67 prediction model could obtain net benefit by predicting Ki67 status of all range risk threshold, and the combined Ki67 prediction model exhibits the highest net benefit (Figure 5C). To further assess the clinical utility of models, clinical impact curves show that the combined Ki67 model has the largest risk threshold range of 0.5-1.0, and the predicted Ki67 status is highly consistent with the actual Ki67 status with risk thresholds ranging between 0.5 and 1.0 (Figure 5D-I).Discussion
To the best of our knowledge, this is the first attempt to establish a Ki67 status prediction model for ICC lesions based on radiomics features. As previous studies, we classified ICC lesions into low Ki67 status group and high Ki67 status group by 25% in our study. However, in the majority of studies on predicting Ki67 status of HCC preoperatively, the cut-off value of low and high Ki67 status is usually selected as 10%-15%. This may be due to the fact that ICC is a much more aggressive cancer than HCC. However, the reason why the Ki67 cut-off value of ICC is higher than that of HCC needs further study. After comparing several models, we found that Clinical+radiomics model, imaging+radiomics and the combined Ki67 prediction model achieve similar predictive efficiency but better than clinical+imaging model, indicating that radiomics features are vital to the prediction of Ki67 status in ICC patients.Conclusion
The combined Ki67 model incorporating clinical feature (HBV), imaging features (arterial rim enhancement on AP and enhancement pattern) and radiomics features (based on T1, T1V and T1D sequences) is a potential biomarker in Ki67 prediction.Acknowledgements
NoneReferences
1. Bergquist A, von Seth E: Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol 2015; 29:221–232.2. Sung H, Ferlay J, Siegel RL, et al.: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71:209–249.
3. J B, Pr G, Sa K, et al.: Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. Journal of hepatology 2014; 60.
4. Qiang Z, Zhang W, Jin S, et al.: Carcinoembryonic antigen, α-fetoprotein, and Ki67 as biomarkers and prognostic factors in intrahepatic cholangiocarcinoma: A retrospective cohort study. Ann Hepatol 2021; 20:100242.
5. Gerdes J, Schwab U, Lemke H, Stein H: Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 1983; 31:13–20.
6. Yang C, Zhang J, Ding M, et al.: Ki67 targeted strategies for cancer therapy. Clin Transl Oncol 2018; 20:570–575.
7. Lambin P, Rios-Velazquez E, Leijenaar R, et al.: Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48:441–446.
Figures
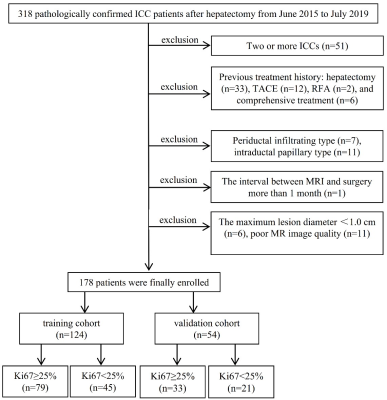
Figure 1. Study flowchart of the enrolled patients.
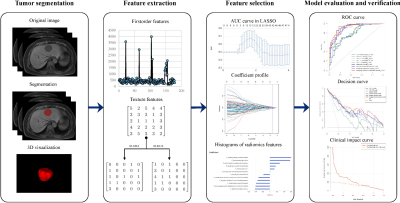
Figure 2. Study flowcharts of radiomics analysis.
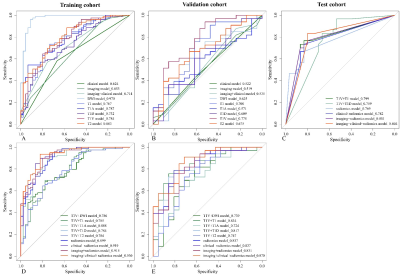
Figure 3. Comparison of receiver operating
characteristics (ROC) curves for Ki67 status prediction in training (A, D),
validation (B, E) and test (C) cohorts by logistic regression.
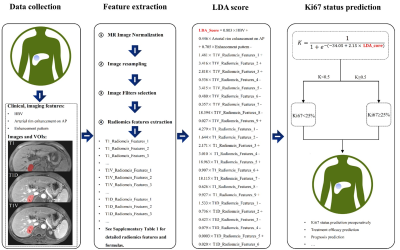
Figure 4. The predictive flowchart of the combined Ki67 prediction model.
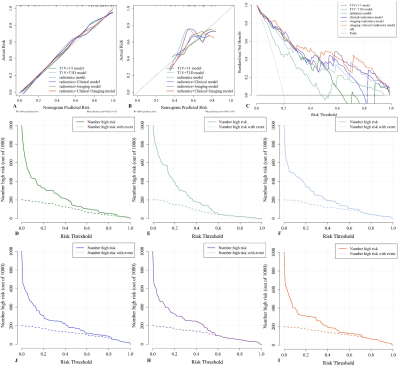
Figure 5. Evaluation and verification of the fusion models. (A and B) Calibration curves of the fusion models in term of agreement between predicted and actual Ki67 status in the training (A) and validation (B) cohort. (C) Decision curves of the fusion models. (D-I) Clinical impact curves of the fusion models. The combined Ki67 model has the largest risk threshold range of 0.5-1.0, and the predicted Ki67 status is highly consistent with the actual Ki67 status with risk thresholds ranging between 0.5 and 1.0.
DOI: https://doi.org/10.58530/2023/1658