1653
Radiomics Nomogram Based on Multi-scale MRI for the Prediction of Microvascular Invasion in Intrahepatic Cholangiocarcinoma1Zhongshan Hospital, Fudan University, Shanghai, China, 2MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: Liver, Radiomics
Microvascular invasion (MVI) is a significant adverse prognostic indicator of intrahepatic cholangiocarcinoma (ICC), and affects the selection of individualized treatment regimen. However, the preoperative imaging-based identification of MVI status is rather difficult. This study sought to establish a multi-sequence and multi-scale MR image-based radiomics nomogram for the presurgical prediction of MVI in ICC.Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second leading primary liver cancer with increasing worldwide incidence in recent years (1–4). MVI is characterized by cancer cell nests within vessels of surrounding liver parenchyma (5), and it is an independent indicator of overall survival (OS) of ICC patients, particularly, early recurrence and poor prognosis (6, 7). Moreover, MVI could influence individualized treatment options, for instance, ICC patients without MVI do not require adjuvant chemotherapy following R0 resection (8). However, the preoperative imaging-based identification of MVI status is rather difficult. Currently, MVI is only identified via postoperative pathology (7–9). Radiomics has been a research hotspot in recent years, which can enhance diagnostic proficiency via high-throughput medical imaging profile selection (10), and can also be applied to preoperative MVI prediction. Pathologically, MVI occurs in peritumoral regions, whereas, in past studies, radiomics features were only retrieved from inside the ICC tumor. Thus, we firstly delineated volumes of interests (VOIs) and retrieved radiomics features from multiple different peritumoral regions of ICC. Totally, our study aimed to develop a radiomics nomogram that integrates clinical, imaging characteristics, and particularly, radiomics features from multi-sequence MR-based multi-scale VOIs for the preoperative prediction of MVI status in mass forming-ICC (MF-ICC).Methods
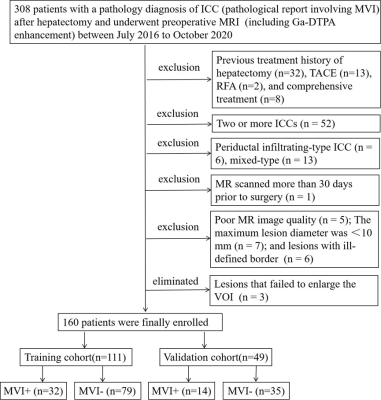
Data Acquisition: 160 patients with single ICC tumors (46 MVI+ and 114 MVI-), who received preoperative MR scans, were arbitrarily separated into training (TC, n = 111) and validation cohorts (VC, n = 49) at a ratio of 7:3. All patients were examined with MR Gd-DTPA DCE scanning. All MR images were retrospectively analyzed by two MVI status-blinded radiologists. VOIs were drawn on six sequences: DWI, T2WI-FS, T1W, T1W-A, T1W-V, and T1W-D. First, the tumor VOI segmentation underwent manual delineation in the ITK-SNAP software by two abdominal radiologists, and this was denoted as VOItumor. Then, two radiologists enlarged the VOItumor by 8, 10, and 12 mm peritumoral regions by using the “Margin” module of the 3D-Slicer software, and then manually removed the volumes outside the hepatic contour, finally, the obtained VOIs were denoted as VOI8mm, VOI10mm, and VOI12mm, respectively. All VOIs were further confirmed by a senior abdominal radiologist. Radiomics features were obtained from images of 6 MR sequences at different peritumoral regions. LASSO was performed to enable derivation of robust and effective radiomics features. Multivariate analysis identified independent clinical and imaging MVI predictors. The newly developed nomogram combined the independent predictors and radiomics features, and its performance was evaluated via the receiver operating characteristic, calibration and decision curves.Results
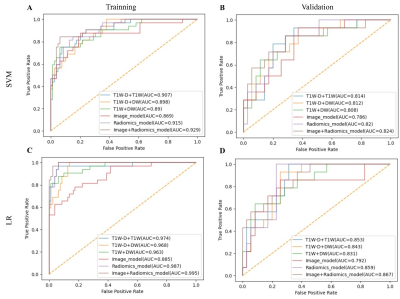
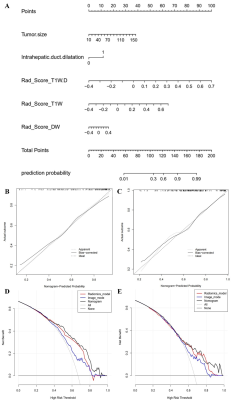
The study flowchart of the enrolled patients and radiomics analysis were exhibited in Figure 1 and Figure 2. Based on multivariate analysis, tumor size (p = 0.01; OR = 1.03, 95% CI: 1.01-1.05) and intrahepatic ductal dilatation (p = 0.02; OR = 3.04, 95% CI: 1.21-7.63) are independent indicators of MVI. And the Image model are constructed with the two independent predictors. In the same sequence, the best predictive efficiencies are obtained from the VOI10mm subgroup. Therefore, the three-sequence combination model (Radiomics model) of DW, T1W, and T1W-D images based on VOI10mm subgroup were constructed and exhibited the ideal predictive efficiencies in both training cohort and validation cohort (AUCTC=0.987, AUCVC=0.859) (Figure 3). The combined Image+Radiomics model achieves excellent and the best predictive efficacy in the TC (AUC = 0.995) and VC (AUC = 0.867). In order to visualize the Image+Radiomics model, we develop a nomogram as an assessment tool (Figure 4A). The nomogram calibration curves demonstrate the goodness-of-fit between the predicted MVI status and the actual MVI status in the TC (Figure 4B) and VC (Figure 4C). The nomogram decision curves exhibit that Image model, Radiomics model and nomogram could obtain net benefits with the risk threshold over 0.3 in the TC (Figure 4D) and 0.5 in the VC (Figure 4E), and the nomogram shows the highest net benefit.Discussion
What we know so far is that we firstly delineated volumes of interests (VOIs) and retrieved radiomics features from multiple different peritumoral regions of ICC. MVI typically presents in the tumor edge, so the radiomics features of the peritumoral tissue must be effectively evaluated. We find that the best scale VOI is VOI10mm, which includes the entire volume of tumor plus the peritumoral area within a region of 10mm from the tumor margin, and the three-sequence combination model based on VOI10mm is regarded as the final radiomics model, and it shows desirable and stable prediction performance. A nomogram combining tumor size, intrahepatic ductal dilatation, and the radiomics model of MR multi-sequence fusion at VOI10 mm may be a predictor of preoperative MVI status in ICC patients. Herein, the preoperative determination of MVI status is of great value in ICC patients, and it holds promises for effective patient management and outcomes estimation.Conclusion
The nomogram combining tumor size, intrahepatic ductal dilatation, and radiomics features of T1W, T1W-D and DW sequences may be an indicator to predict presurgical MVI status in ICC patients.Acknowledgements
NoneReferences
1. Squadroni M, Tondulli L, Gatta G, Mosconi S, Beretta G, Labianca R: Cholangiocarcinoma. Crit Rev Oncol Hematol 2017; 116:11–31.
2. Sung H, Ferlay J, Siegel RL, et al.: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA CANCER J CLIN 2021; 71:41.
3. Utada M, Ohno Y, Tamaki T, Sobue T, Endo G: Long-term trends in incidence and mortality of intrahepatic and extrahepatic bile duct cancer in Japan. J Epidemiol 2014; 24:193–199.
4. Bergquist A, von Seth E: Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol 2015; 29:221–232.
5. Cong W-M, Bu H, Chen J, et al.: Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol 2016; 22:9279–9287.
6. Surov A, Pech M, Omari J, et al.: Diffusion-Weighted Imaging Reflects Tumor Grading and Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer 2021; 10:10–24.
7. Shao C, Chen J, Chen J, Shi J, Huang L, Qiu Y: Histological classification of microvascular invasion to predict prognosis in intrahepatic cholangiocarcinoma. Int J Clin Exp Pathol 2017; 10:7674–7681.
8. Tsukamoto M, Yamashita Y-I, Imai K, et al.: Predictors of Cure of Intrahepatic Cholangiocarcinoma After Hepatic Resection. Anticancer Res 2017; 37:6971–6975.
9. Zhou Y, Wang X, Xu C, et al.: Mass-forming intrahepatic cholangiocarcinoma: Can diffusion-weighted imaging predict microvascular invasion? J Magn Reson Imaging 2019; 50:315–324.
10. Lambin P, Leijenaar RTH, Deist TM, et al.: Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017; 14:749–762.
Figures