1647
Prediction of Histological Subtypes Using Machine-Learning Model Based on UTE-MRI in Non-Small Cell Lung Cancer:A comparative study with CT1Department of Medical Imaging, Henan University People’s Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 2Department of Medical Imaging, Zhengzhou University People’s Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 3Department of Medical Imaging, Xinxiang Medical University Henan Provincial People’s Hospital, Zhengzhou, China, 4Central Research Institute, UIH Group, Shanghai, China, 5Beijing United Imaging Research Institute of Intelligent Imaging, UIH Group, Beijing, China
Synopsis
Keywords: Cancer, Radiomics
Three-dimensional ultrashort echo time (3D-UTE) is a novel MRI technique, which yields similar diagnostic results as conventional pulmonary computed tomography (CT). Our results showed that the predication model based on clinical factors and 3D-UTE radiomics features could noninvasively assess the subtype of in non-small cell lung cancer. Compared with the CT model, it has similar diagnostic efficiency but less radiation, which is expected to provide new ideas for related research.Introduction
More than 80% of lung cancer cases are non-small cell lung cancer (NSCLC) and the subtype of NSCLC can affect the formulation of the treatment plan [1]. Three-dimensional ultrashort echo time (3D-UTE) is a novel MRI technique, which not only does not have ionizing radiation but also yields similar diagnostic results as conventional pulmonary computed tomography (CT) [2]. Radiomics is a key innovation in medical image analysis that enables high-throughput extraction of quantitative features from medical images [3]. To our knowledge, there is no relevant study involving 3D-UTE radiomics models in the field of lung lesion evaluation that has been reported thus far. Hence, the purpose of this study was to use machine learning methods to develop an UTE-MRI model combining clinical factors and 3D-UTE radiomics features to differentiate adenocarcinoma (AC) from squamous cell carcinoma (SCC), offering a potential reference for the clinical management of NSCLC.Material and Methods
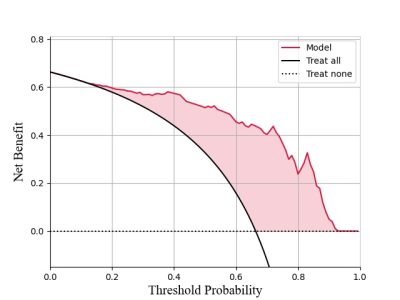
A total of 107 NSCLC patients (training, 74 cases; test, 33 cases) were enrolled in this study. The diagnostic CT (tube current 10 mA, voltage 120 kV, rotation time 0.8 s, pitch 0.6125, collimation 40 mm) was performed first. Then a 3.0 T MR system (uMRI 790, UIH, Shanghai, China) with a 12-channel phased-array body coil was performed. A respiratory-gated axial 3D-UTE pulse sequence was obtained according to the following parameters: TR = 2.6 ms, TE = 0.16 ms, flip angle = 3°, slice thickness = 2 mm, matrix = 456 × 456, voxel size = 0.767 × 0.767 × 2 mm3, acquisition time = 5 min 26 s. The uAI software (United Imaging Intelligence, Shanghai, China) was used for volume of interest region (VOI) segmentation and radiomics feature extraction.The Mann‒Whitney U test, least absolute shrinkage and selection operator (LASSO) logistic regression, and SelectKBest were performed to select the most relevant features. Partial least squares discriminant analysis (PLS-DA), logistic regression (LR), and support vector machine (SVM) were applied in model establishment [4, 5] (Figure.1).Statistical analyses were performed with Python (Version 3.10; Python Software Foundation) software. The Mann-Whitney U test and chi-square test were used to analyse the differences of continuous variables and the differences of categoric variables, respectively. The ROC was employed to quantify the diagnostic efficacy, and the differences were assessed using DeLong analysis. The calibration degree of the model was evaluated by the Hosmer-Lemeshow (H-L) test and presented by calibration curve. The decision curve analyses (DCA) was applied to calculate the clinical net benefit.
Results
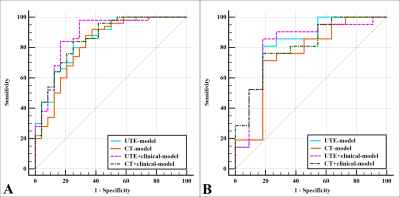
A prediction model based on PLS-DA, consisting of four clinical factors and six 3D-UTE radiomics features, was used as the final model for UTE-MRI. The AUCs of this model were 0.882 and 0.823 in the training and test sets, respectively, which not only showed different degrees of improvement over individual models such as clinical and 3D-UTE (AUC-training = 0.845, 0.828, AUC-test = 0.792, 0.768, respectively), but also achieved the similar diagnostic efficacy as the optimal CT model consisting of four clinical factors and six CT radiomics features (AUC-training = 0.858, AUC-test = 0.810) (Figure.2). The calibration curves and DCA indicated good consistency (C-index, 0.882) and clinical utility of this model, respectively (Figure.3).Discussion
This study developed a UTE-MRI model and compared it with clinical, 3D-UTE, and CT models. The results showed that the diagnostic efficacy of the UTE-MRI model not only was improved to different degrees compared with the clinical, and 3D-UTE models, but also achieved the similar diagnostic efficacy as the CT model consisting of four clinical factors and six CT radiomics features, which can provide a new idea with less radiation burden for relevant clinical diagnosis and treatment. Classification method options are decisive in affecting the performance of machine learning classification, and different classification methods have their own strengths and weaknesses [6]. Therefore, applying as many classification methods as possible to filter out the most appropriate prediction models when conditions permit may still be the most effective machine learning modeling approach at present.Conclusion
The UTE-MRI model based on clinical factors, and 3D-UTE radiomics features using machine learning methods could noninvasively assess the subtype of NSCLC. Compared with the CT model, it has similar diagnostic efficiency but less radiation, which is expected to provide new ideas for related research.Acknowledgements
The National Key R&D Program of China (2017YFE0103600), the National Natural Science Foundation of China (81720108021 and 31470047), the Zhongyuan Thousand Talents Plan Project - Basic Research Leader Talent (ZYQR201810117), the Zhengzhou Collaborative Innovation Major Project (20XTZX05015), the Key Project of Henan Province Medical Science and Technology Project (LHGJ20190602), and the Henan provincial science and technology research projects (212102310689).References
1. Ettinger DS, Wood DE, Aisner DL et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw. 2021; 19:254-266.
2. Ohno Y, Koyama H, Yoshikawa T et al. Pulmonary high-resolution ultrashort TE MR imaging: Comparison with thin-section standard- and low-dose computed tomography for the assessment of pulmonary parenchyma diseases. J Magn Reson Imaging. 2016; 43:512-532.
3. Thawani R, McLane M, Beig N, et al. Radiomics and radiogenomics in lung cancer: A review for the clinician. Lung Cancer. 2018;115:34-41.
4. Brereton R G , Lloyd G R. Partial least squares discriminant analysis: taking the magic away. Journal of Chemometrics. 2014; 28:213-225.
5. Zhou Y, Ma XL, Zhang T, Wang J, Zhang T, Tian R. Use of radiomics based on 18F-FDG PET/CT and machine learning methods to aid clinical decision-making in the classification of solitary pulmonary lesions: an innovative approach. Eur J Nucl Med Mol Imaging.2021 48:2904-2913.
6. Qian Z, Li Y, Wang Y et al (2019) Differentiation of glioblastoma from solitary brain metastases using radiomic machine-learning classifiers. Cancer Lett 451:128-135.
Figures