1643
High resolution rotating frame relaxation mapping with T-NORDIC1CMRR, Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Keywords: Quantitative Imaging, Brain
Current whole-brain implementations of rotating frame relaxation (RFR) mapping are generally limited to moderate spatial resolutions due to acquisition time limitations. We propose using transform domain NORDIC, T-NORDIC, to increase the quality of RFR maps at both moderate and high spatial resolution. The results show clear improvements in terms of fitting reliability for estimation of adiabatic T1ρ and T2ρ, and especially Relaxation Along a Fictitious Field in rotating frame of rank n=4 (RAFF4) after applying NORDIC denoising. The improvements in map quality can be leveraged to reduce acquisition times in clinical applications.Introduction
Adiabatic T1ρ, T2ρ, (1) and RAFFn (2) are rotating frame relaxation constants that are sensitive to spin dynamics in the kHz range, and have been shown to provide useful markers for probing tissue integrity. Whole-brain implementations of RFR mappings have been safely and robustly used in clinical settings (see e.g. (3,4)) at moderate spatial resolution (e.g. 1.6 mm x 1.6 mm x 3.6 mm). However, for precise morphometrical examinations especially in pathological states, higher spatial image resolutions are desirable. Here we propose to improve the spatial resolution while maintaining a robust estimation of relaxation times by using a transform domain locally low-rank method called T-NORDIC, a modified version of NOise Reduction with DIstribution Corrected (T-NORDIC) PCA denoising (5), in which a unitary transform is utilized to better leverage low-rank properties of patches while preserving noise distributions.Method
Brain images of four healthy subjects (age, mean ± SD = 45.5 ± 22 years, males) were acquired on a 3T Prisma Siemens scanner equipped with 64-channel receive coil. T1ρ and T2ρ were obtained with gradient echo sequence with magnetization preparation using hyperbolic secant HS1 pulses, pulse duration = 6 ms, bandwidth BW = 1.3 kHz, peak power ω1max/(2π) = 800 Hz, and number of pulses = 0, 4, 8, 12, 16 phase cycled according to MLEV-4. For RAFF4, the durations of P-packets was 4.52 ms, number of P-packets set to 0, 8, 16, 32, and with ω1max/(2π) = 327 Hz; two RAFF4 consecutive acquisitions were conducted without and with a global inversion.With the same field of view, data acquisition was performed with two different spatial resolutions: low resolution (voxel size = 1.6 × 1.6 × 3.6 mm3, 30 slices), and high resolution (voxel size = 1.25 x 1.25 x 2 mm3, 54 slices). Anatomical 3D T1weighted images (using MPRAGE) have been also acquired for each subject as reference volumes. In T-NORDIC non-asymptotic random matrix characterization of the thermal noise (as introduced in NORDIC) was obtained and applied using the FFT as unitary transformation.
Subject-specific atlases were obtained with the full segmentation algorithm implemented in FreeSurfer (https://surfer.nmr.mgh.harvard.edu/) and aligned quantitative maps using ANTS (http://stnava.github.io/ANTs/). T1ρ and T2ρ maps were estimated from a mono-exponential fitting, whereas TRAFF4 was obtained using a bi-exponential fitting procedure; the goodness of parameter fitting was evaluated for each voxel as 95% confidence interval (CI). Regional parameter means, spatial coefficients of variation (CoV), and CI values were extracted in white (WM) and cortical grey matter (GM) and in a representative smaller subcortical region (caudate nucleus, CN).
Results
Figure 1 shows the denoising effect of T-NORDIC on quantitative T1ρ (a), T2ρ (b), and RAFF4 (c) maps of one representative subject. T-NORDIC improvement is especially evident in the high-resolution acquisition when looking at local brain areas, as also illustrated by zoomed images in the last row. Maps of CI of fitted parameters show that, for both spatial resolutions, the use of T-NORDIC leads to a more precise estimation of the quantitative parameters as reflected in lower CI values (Figure 2 based on subject data, and Figure 3 for mean across subjects). Importantly, when extracting mean values in regions of interest, the use of T-NORDIC does not alter the estimation of T1ρ, T2ρ (Figure 3a, left panels), and RAFF4 (figure 3b, left panels) as compared to the state-of-the-art settings, while improving CI and CoV in high resolution acquisition.Discussion
Our study demonstrates that the use of T-NORDIC denoising allows high-resolution RFR mapping of the brain without compromising image quality. Importantly, T-NORDIC increases the reliability of parameter determination without altering the quantitative estimation of the relaxation times. These results draw a path for pushing RFR mappings to high resolution in clinical settings.Acknowledgements
This work was supported by NIH grant P41 EB027061.References
1. Mangia S, Liimatainen T, Garwood M, Michaeli S. Rotating frame relaxation during adiabatic pulses versus conventional spin-lock: simulations and experimental results at 4T. Magn Reson Imaging 2009;27:1074–1087 doi: 10.1016/j.mri.2009.05.023.
2. Liimatainen T, Sorce DJ, O’Connell R, Garwood M, Michaeli S. MRI contrast from relaxation along a fictitious field (RAFF). Magn Reson Med 2010;64:983–994 doi: 10.1002/mrm.22372.
3. Mangia S, Svatkova A, Mascali D, et al. Multi-modal Brain MRI in Subjects with PD and iRBD. Front Neurosci 2017;11:709 doi: 10.3389/fnins.2017.00709.
4. Filip P, Svatkova A, Carpenter AF, et al. Rotating frame MRI relaxations as markers of diffuse white matter abnormalities in multiple sclerosis. NeuroImage: Clinical 2020;26:102234 doi: 10.1016/j.nicl.2020.102234.
5. Moeller S, Pisharady PK, Ramanna S, et al. NOise reduction with DIstribution Corrected (NORDIC) PCA in dMRI with complex-valued parameter-free locally low-rank processing. NeuroImage 2021;226:117539 doi: 10.1016/j.neuroimage.2020.117539.
Figures
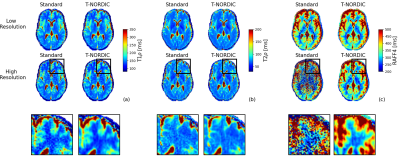
Figure 1. T1ρ (a), T2ρ (a), and RAFF(c) maps from one representative subject in both spatial resolutions and with and without T-NORDIC denoising. T1ρ and T2ρ were estimated from mono-exponential voxel-wise fitting while RAFF from bi-exponential fitting. The last row shows a zoom region of the high resolution data, showing the local effects of T-NORDIC denoising.
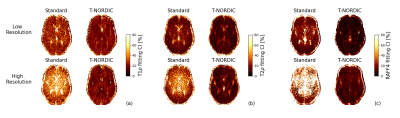
Figure 2. 95% confidence intervals (CI) of the estimated parameters, expressed as percentages, from one representative subject in both spatial resolutions and with and without T-NORDIC denoising for T1ρ (a) and T2ρ (b) and RAFF (c). Lower CI indicates higher precision in parameter estimation.
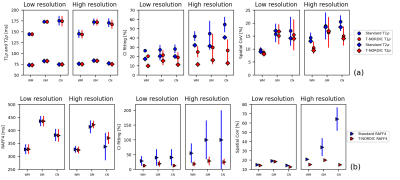
Figure 3. Mean regional values averaged across the four subjects of T1ρ and T2ρ (a) and RAFF (b) for the different spatial resolutions (first and second panels), of the parameter CI (third and fourth panels) and of the spatial coefficient of variation (CoV, last two panels). Values were extracted in three representative regions: white matter (WM), cortical grey matter (GM) and caudate nucleus (CN).