1641
High Resolution Luminal Water Imaging for Prostate Cancer Diagnosis and Grading
Maryam Mohtajeb1, Erin MacMillan1,2, Guillaume Gilbert2, and Piotr Kozlowski1,3,4
1MRI Research Center, University of British Columbia, Vancouver, BC, Canada, 2MR Clinical Science, Philips Healthcare, Mississauga, ON, Canada, 3Department of Radiology, University of British Columbia, Vancouver, BC, Canada, 4Department of Urologic Sciences, University of British Columbia, Vancouver, BC, Canada
1MRI Research Center, University of British Columbia, Vancouver, BC, Canada, 2MR Clinical Science, Philips Healthcare, Mississauga, ON, Canada, 3Department of Radiology, University of British Columbia, Vancouver, BC, Canada, 4Department of Urologic Sciences, University of British Columbia, Vancouver, BC, Canada
Synopsis
Keywords: Quantitative Imaging, Prostate, Quantitative Imaging, Pulse Sequence Design
Current prostate cancer diagnosis and grading techniques have several limitations, which dictates further endeavors toward developing a more accurate approach. Luminal water imaging (LWI) has demonstrated superior diagnostic accuracy than the current multiparametric MRI standardized protocol. In this new application, we further optimized LWI acquisition by decreasing the in-plane resolution without sacrificing SNR by applying two different reduced field-of-view techniques. Our new sequences provide improved in-plane resolution to delineate anatomical details and provide high-quality data for LWI analysis. This new LWI acquisition could replace the lengthy multiparametric MRI that suffers from technical challenges, inferior diagnosis accuracy, and a required contrast agent injection compared to LWI.Introduction
Prostate cancer is among the leading causes of cancer-related deaths in North American males. Early and accurate diagnosis is critical for successful treatment. However, considering the heterogeneous and multifocal nature of prostate cancer, accurate assessment of tumor grade and extent of invasion before surgical removal is currently impossible. More accurate, non-invasive diagnostic techniques are needed to identify and grade malignant foci. Prostate cancer management has improved recently due to advances in multiparametric MRI (mp-MRI) combining multiple MRI-based techniques such as T2-weighted, Diffusion Weighted Imaging, Dynamic Contrast-Enhanced MRI, and/or MR Spectroscopy to improve diagnostic power. Though mp-MRI is increasingly being recognized as a diagnostic standard, it suffers from missed detection of up to 15% of clinically significant cancers, low accuracy in tumor grading, contrast agent injection, technical challenges with diffusion imaging in patients with metallic implants, and lengthy examination times. A novel technique based on fitting the T2 decay from multi-spin-echo images, called Luminal Water Imaging (LWI), has recently been developed 1-4. LWI has shown higher accuracy in cancer detection, a significantly higher correlation with the Gleason score than the mp-MRI 3, and higher diagnostic accuracy than the current reporting standards 4. In addition, LWI avoids the need for contrast agent injections. One of the limitations of the previous LWI sequence is a relatively low in-plane resolution, resulting in the lack of anatomical details that radiologists rely on for diagnosis. To make the LWI more clinically applicable, we propose to develop a novel LWI acquisition protocol to increase the in-plane resolution. In this work, an LWI pulse sequence with the submillimeter in-plane resolution was implemented and tested on a normal volunteer.Method
LWI was acquired on a 3T Philips Ingenia Elition X with a 3D gradient and spin echo (GRASE) sequence with 64 spin echoes. Acquisition time was shortened by reducing the number of phase-encoding steps by limiting the field of view (FOV) to the extent of the prostate. Two techniques were used to reduce the phase FOV: Regional saturation (REST) foldover suppression and Zoom (inner-volume selection). In Zoom, the sample volume is imaged as an intersection of the overlap between the 90° pulse in the slice selective axis and the 180° pulse in the phase encoding axis. For both techniques, additional REST slabs were added in the foot-head (FH) direction to suppress the abundance of outer-volume signal projecting back into the image. To decrease the observed free induction decay (FID) artifacts with the REST technique, crusher gradients were programmed on the frequency encoding axis. Other scan parameters were as follows: a) REST: Slices: 24 transverse; foldover direction= Right-Left (RL); FOV: Anteroposterior (AP)×RL×FH= 240×120×48 mm3; Acquisition voxel size: AP×RL×FH= 1×1.6×4 mm3; Reconstruction voxel size: AP×RL×FH= 0.5×0.5×2 mm3; EPI factor =5; TR/ΔTE = 3074/25 ms; Number of signal averages (NSA)=1; Total scan duration= 12min:45s. b) Zoom: Similar to the REST except the acquisition voxel size: AP×RL×FH= 1×1.8×4 mm3; Reconstruction voxel size: AP×RL×FH= 0.8×0.8×2 mm3; and scan duration=15 min:44s.Results
The acquired images, their corresponding luminal water fraction (LWF) maps, and the T2 decay curve/T2 distribution for a point in the peripheral zone of the prostate are presented in Figures 1-3.Discussion
Our results demonstrate that we successfully improved the in-plane resolution within imaging times of about 15 and 12 minutes at the limit of being clinically practical. Furthermore, including crushers for the REST foldover suppression shows promising results regarding reducing the FID artifacts and making the LWF maps more continuous with minimal effect on the T2 decay curve and T2 distribution. Implementing FID reduction crushers for the Zoom application is an ongoing effort that may improve the quality of both image and LWF maps.Conclusion
Reduced FOV acquisition for LWI improves the in-plane spatial resolution. Both Zoom and REST offer similar gains in resolution, though Zoom resulted in an increase in scan time. Adding crushers to reduce the FID signals at each spin echo improved the spatial continuity of the LWF maps without impacting the T2 decay data and fitting. If implemented on clinical MRI scanners, our sequence provides a more accurate diagnosis based on anatomy combined with LWI data5 facilitating patient risk stratification with an improved management course and can become a new imaging standard.Acknowledgements
This work was supported by a research grant from the Vancouver Coastal Health Research Institute (VCHRI).References
- Sabouri S, SD Chang, R Savdie, J Zhang, EC Jones, SL Goldenberg, PC Black, and P Kozlowski, "Luminal Water Imaging: A New MR Imaging T2 Mapping Technique for Prostate Cancer Diagnosis", Radiology,2017;284:451-459.
- Sabouri S, L Fazli, SD Chang, R Savdie, EC Jones, SL Goldenberg, PC Black, and P Kozlowski, "MR measurement of luminal water in prostate gland: Quantitative correlation between MRI and histology", J Magn Reson Imaging,2017;46:861-869.
- Sabouri S, SD Chang, SL Goldenberg, R Savdie, EC Jones, PC Black, L Fazli, and P Kozlowski, "Comparing diagnostic accuracy of luminal water imaging with diffusion-weighted and dynamic contrast-enhanced MRI in prostate cancer: A quantitative MRI study", NMR Biomed,2019;32:e4048.
- Sabouri S, Chang S, Pang E, Mohammedeid R, Jones E, Goldenberg SL, Black P, Kozlowski P, “Comparing the Diagnostic Accuracy of Luminal Water Imaging versus PI-RADSv2.1 in Detection of Prostate Cancer”, ISMRM & SMRT Virtual Conference & Exhibition, 8-14 August 2020, abst. #709.
- Ip CJX, Chang SD, Sabouri S, Yung A, Reinsberg S, Kozlowski P, “Enhanced T2-weighted images using Luminal Water Imaging and U-Net based segmentation for prostate cancer diagnosis”, Joint Annual Meeting ISMRM-ESMRMB& SMRT 31st Annual Meeting, 07-12 May 2022, London, England, UK.
Figures
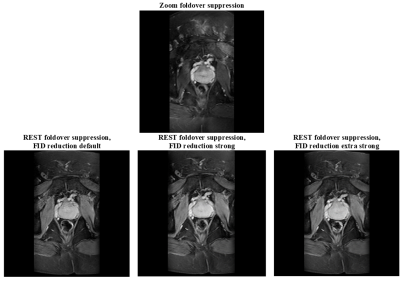
Figure
1: Echo2/Slice17 of Zoom foldover
suppression and echo2/slice1 of regional saturation (REST) foldover suppression
with various strengths for the free induction decay (FID) reduction crushers
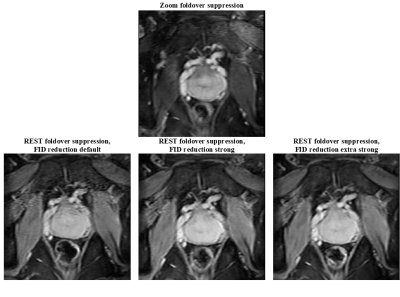
Figure
2: Echo2/Slice17 of
Zoom foldover suppression and echo2/slice1 of regional saturation (REST)
foldover suppression with various strengths for the free induction decay (FID) reduction
crushers in a zoom-in view
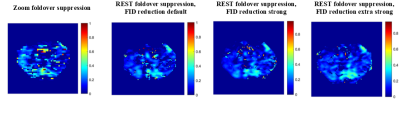
Figure
3: LWF maps for a selected slice of
zoom foldover suppression and regional saturation (REST) foldover suppression
with various strengths for the free induction decay (FID) reduction crushers
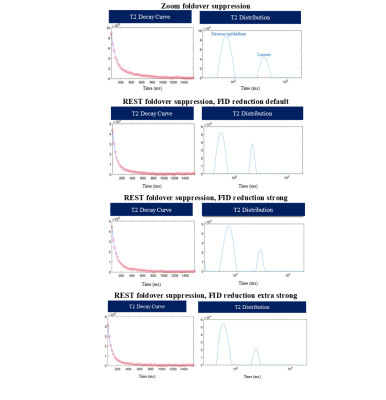
Figure
4: T2 decay curve and T2
distribution for a point in the peripheral zone of the prostate for zoom
foldover suppression and regional saturation (REST) foldover suppression with
various strengths for the free induction decay (FID) crushers
DOI: https://doi.org/10.58530/2023/1641