1637
Novel water-fat separation model for multi-echo bipolar readout1Biomedical Imaging Center, Pontificia Universidad Catolica de Chile, Santiago, Chile, 2i-Health Millenium Institute in Intelligent Healthcare Engineering, Santiago, Chile, 3Institute for Mathematical and Computational Engineering, Pontificia Universidad Catolica de Chile, Santiago, Chile, 4Institute for Biological and Medical Engineering, Pontificia Universidad Catolica de Chile, Santiago, Chile, 5Department of Electrical Engineering, School of Engineering, Pontificia Universidad Catolica de Chile, Santiago, Chile, 6School of Electrical Engineering, Pontificia Universidad Catolica de Valparaiso, Valparaiso, Chile, 7Radiology Department, School of Medicine, Pontificia Universidad Catolica de Chile, Santiago, Chile
Synopsis
Keywords: Quantitative Imaging, Fat
This work proposes a new model for solving the water-fat separation problem with bipolar acquisition, which allows us for reducing the acquisition time and computing accurate PDFF without any special concerns about phase corrections. We solve jointly two water-fat separation problems for even and odd echoes, under the assumption that the concentrations are different, but considering the same field map and $$$R_2^*$$$. Bland Altman plots and linear regression show excellent performance for PDFF estimation in 8 healthy volunteers.Introduction
Water-fat separation techniques estimate the water and fat concentrations jointly with the field map and $$$R_2^*$$$1. Although unipolar readout is a very robust sequence for Proton Density Fat Fraction (PDFF) estimation, a bipolar readout shortens the scan time by reducing the echo spacing2. This is useful for breath-hold acquisitions typically used in the liver. However, using bipolar readouts becomes challenging due to the phase inconsistencies between even and odd echoes. Some of them can be corrected by shifting the k-space center of even echoes2, however other effects, such as concomitant gradients and eddy currents are harder to overcome3. They have been addressed using an extra non-linear phase term that changes its polarity between even and odd echoes4 and interleaved acquisitions5. In this work we propose a new method for solving the water-fat separation problem with bipolar acquisition, allowing to compute accurate PDFF without any special concerns about phase corrections.Methods
We jointly solve two water-fat separation problems for even and odd echoes, assuming that the concentrations are different but the field map and $$$R_2^*$$$ are identical. The conventional model of water-fat separation problem can be defined as follows1, $$ s(t) = (\rho_w + \rho_f \sum_{p=1}^{n_p} \omega_p \mathrm{e}^{i2\pi f_p t}) \mathrm{e}^{i2 \pi \xi t} ,$$where $$$\rho_w$$$ and $$$\rho_f$$$ are the complex water and fat concentrations, $$$f_p$$$ and $$$\omega_p$$$ the frequencies and relative amplitudes of the pre-calibrated $$$n_p$$$-peak fat spectrum6, and $$$\xi = \phi + i 2\pi R_2^*$$$, where $$$\phi$$$ and $$$R_2^*$$$ denotes the field map and the signal decay.
Considering $$$n$$$ echo times, the matrix form of the previous problem is given by $$s = W(\xi)M\rho,$$ where $$$s$$$ is the signal vector, $$$W(\xi) = \mathrm{diag}(\mathrm{e}^{i2\pi t_1}, …, \mathrm{e}^{i2\pi t_n})$$$, $$$\rho$$$ is the concentration vector and,
$$M := \begin{bmatrix} 1 & \sum\limits_{p=1}^{n_p} \omega_{p} \text{e}^{2\pi i f_p t_1} \\ \vdots & \vdots \\ 1 & \sum\limits_{p=1}^{n_p} \omega_{p} \text{e}^{2\pi i f_p t_{n}}\end{bmatrix}.$$
Considering the aforementioned assumptions, the matrix $$$M$$$ can be reformulated as follows,
$$M := \begin{bmatrix} 1 & 0 & \sum\limits_p^{n_p} \omega_{p} \text{e}^{i 2\pi f_p t_1} & 0 \\ 0 & 1 & 0 & \sum\limits_{p=1}^{n_p} \omega_{p} \text{e}^{i 2\pi f_p t_2} \\ 1 & 0 & \sum\limits_p^{n_p} \omega_{p} \text{e}^{i 2\pi f_p t_3} & 0\\ \vdots & \vdots & \vdots & \vdots \\ 0 & 1 & 0 & \sum\limits_{p=1}^{n_p} \omega_{p} \text{e}^{i 2\pi f_p t_n} \end{bmatrix},$$
and $$$\rho=[\rho_w^+ \rho_w^- \rho_f^+ \rho_f^-]^T$$$, with $$$ +$$$ and $$$ - $$$ denoting the readout gradient polarity.
We solve the following optmization problem $$\begin{equation*} \begin{aligned} & \underset{\xi}{\mathrm{min}} & & \frac{1}{2}\sum\nolimits_{v \in V} ||{P(\xi_v)s}||_2^2 + \beta_1 \mathrm{TV}(\text{Im}(\xi))\\ & \mathrm{s.t.} & & \forall\,v\in V:\,\, ||{\nabla \text{Re}(\xi_v)}||_2 \leq \varepsilon_\Phi,\,\, 0 \leq \text{Im}(\xi_v) \end{aligned}\end{equation*},$$
where, $$$P(\xi) := W(\xi) (I_n - M M^+) W(-\xi)$$$, $$$M^+$$$ is the pseudo-inverse of $$$M$$$, $$$v$$$ denotes one voxel, the first constraint is an smoothing term for the field map to prevent swaps and the second one is the $$$R_2^*$$$ non-negativity constraint. This problem can be solve using Gauss Newton Trust Region7, combining ADMM8 and FISTA9.
Concentrations are obtained by solving the least square problem $$$ \rho(\xi) = M^+ W(-\xi) s$$$. Increasing the number of concentrations decreases the SNR of the estimations, deteriorating the PDFF. This is compensated by using all the concentrations to estimate the PDFF,
$$PDFF = \frac{|\rho_f^+| + |\rho_f^-|}{|\rho_f^+| + |\rho_f^-| + |\rho_w^+| + |\rho_w^-|}.$$
To validate our proposal, we prospectively acquired 8 healthy volunteers in a 1.5T Philips Achieva scanner. As ground truth, we used a 6-echoes gradient echo unipolar acquisition with $$$\Delta$$$TE/TE1 2.1/1.3, flip angle 10°, TR 30ms, voxel size 2x2x8 mm. Water/fat separation was performed using graph cuts10. Bipolar acquisitions were identical to the latter, except for the reduced $$$\Delta$$$TE to 1.1 ms.
For statistical analysis we use linear regression, correlation and Bland Altman (BA) plots of the median PDFF, considering two regions of interest (ROIs), in the left and right hepatic lobes.
Results
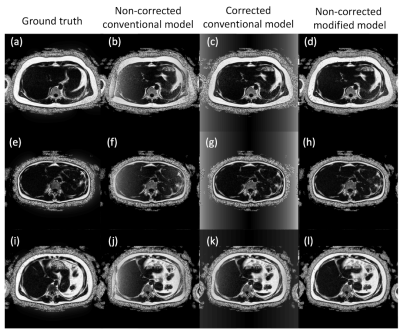
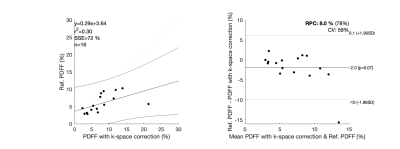
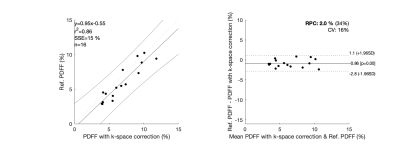
Non-corrected data lead to wrong PDFF estimations (Fig.1 (b), (f), (j)). Results with k-space correction (Fig. 1 (c), (g), (k)) showed an improvement in PDFF appealing, however, the performance was quantitively poor, with an $$$r^2$$$ correlation coefficient of 0.30 and BA plots showed a considerable bias and CV of 59%. Our new water-fat model (Fig. 1 (d), (h), (l)) improved the $$$r^2$$$ correlation coefficient to 0.86, reduced the bias to 0.86% of fat, showed a good LOA, and the CV was 16%.Discussion
Our new water-fat separation model allows us for computing accurate PDFF without any concern about the phase inconsistencies produced by bipolar readout, and therefore, achieving shorter scan times assuring the necessary number of echoes for a good quantification11. Instead of adding non-linear unknowns to the problem, we introduced additional concentrations, which are easier to estimate. In that sense, all the phase inconsistencies are expressed by the phase of these concentrations. A constraint that our model missed is that the concentrations have the same magnitude. We have not found a straightforward way to impose that constraint. The major cost paid by our model is the SNR decay for introducing more variables. We compensate that by combining the magnitudes of all variables in the final PDFF estimation.Conclusion
Novel water-fat separation model allows for obtaining accurate PDFF with bipolar readout acquisitions and without any special concern about the phase of even and odd echoes. Future work includes analyzing subjects with higher-fat liver content.Acknowledgements
This work was funded by ANID – Millennium Science Initiative Program – ICN2021_004, ANID Fondecyt 1181057, ANID Fondecyt 1191710, ANID Fondecyt 1231535, ANID–PIA–ACT 192064 and PUENTE grant 2022-14 VRI, PUC. C.A. was partially funded by ANID Fondecyt Postdoctorado 2019 #3190763. C.S-L was funded by ANID Fondecyt 1211643 and Centro Nacional de Inteligencia Artificial CENIA, FB210017, BASAL, ANID. J.M was funded by the National Agency for Research and Development (ANID) / Scholarship Program / DOCTORADO BECAS CHILE/2020 – 21210665. H. M. thankfully acknowledges thee financial support of the project ANID - FONDECYT Postdoctorado #3220266.References
1. Huanzhou Yu, Charles A. McKenzie, Ann Shimakawa, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. Journal of Magnetic Resonance Imaging 26:1153-1161 (2007).
2. Wenmiao Lu, Huanzhou Yu, Ann Shimakawa, Marcus Alley, Scott B. Reeder and Brian A. Hargreaves. Water–Fat Separation with Bipolar Multiecho Sequences. Magnetic Resonance in Medicine 60:198–209 (2008).
3. Huanzhou Yu, Ann Shimakawa, Charles A. McKenzie, Wenmiao Lu, Scott B. Reeder, R. Scott Hinks, and Jean H. Brittain. Phase and Amplitude Correction for Multi-Echo Water–Fat Separation with Bipolar Acquisitions. Journal of Magnetic Resonance Imaging 31:1264–1271 (2010).
4. Pernilla Peterson and Sven Månsson. Fat Quantification Using Multiecho Sequences with Bipolar Gradients: Investigation of Accuracy and Noise Performance. Magnetic Resonance in Medicine 71:219–229 (2014).
5. Abraam S. Soliman, Curtis N. Wiens, Trevor P. Wade and Charles A. McKenzie. Fat Quantification Using an Interleaved Bipolar Acquisition. Magnetic Resonance in Medicine 75:2000–2008 (2016).
6. Gavin Hamilton, Takeshi Yokoo, Mark Bydder, Irene Cruite, Michael E Schroeder, Claude B Sirlin and Michael S Middleton. In vivo characterization of the liver fat ¹H MR spectrum. NMR Biomed 24(7):784-90 (2011).
7. Jorge Nocedal, Stephen J. Wright. Numerical Optimization. Springer New York; 2nd ed. (2006).
8. Stephen Boyd, Neal Parikh, Eric Chu, Borja Peleato, Jonathan Eckstein. Distributed optimization and statistical learning via the alternating direction method of multipliers. (2010).
9. Amir Beck, Marc Teboulle. A fast iterative shrinkage-thresholding algorithm for linear inverse problems. SIAM Journal on Imaging Sciences 2 (2009).
10. Diego Hernando, P. Kellman, J.P. Haldar, Z.P. Liang. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magnetic Resonance in Medicine. 63:79-90 (2010).
11. Benjamin Henninger, Jose Alustiza, Maciej Garbowski, Yves Gandon. Practical guide to quantification of hepatic iron with MRI. European Radiology. 30 (2020).
Figures