1635
Preliminary investigation of a rapid proxy measure of T2* in cartilage using a double echo uTE
Aditya K Subramanian1,2, Lauren Watkins2, Garry G Gold2, Feliks Kogan2, and Marco Barbieri2
1UC-Berkeley, BERKELEY, CA, United States, 2Department of Radiology, Stanford University, Stanford, CA, United States
1UC-Berkeley, BERKELEY, CA, United States, 2Department of Radiology, Stanford University, Stanford, CA, United States
Synopsis
Keywords: Quantitative Imaging, Quantitative Imaging
Ultrashort echo time (uTE) T2* mapping of articular cartilage is sensitive to changes in components of cartilage with short T2* such as water bound to proteoglycans and collagen. T2* mapping requires acquiring multiple echoes and fitting the signal intensities to a monoexponential decay model to estimate a single T2*. We propose a ratio based proxy for T2*, the Free Water Index (FWI). Our exploratory study showed high correlations between the index and T2* in vivo, supporting the potential for our index to reduce scan time while retaining information from T2*.Introduction
Osteoarthritis (OA) is a leading cause of disability and chronic pain worldwide without any disease-modifying therapy. Early diagnosis is key for the prevention and treatment of OA before progression into irreversible stages. The transverse relaxation time (T2) has been shown to be sensitive to macromolecular changes in collagenous tissues 1, 2. However, conventional T2 mapping cannot capture the signal from components with very short echo times (less than a few ms) such as the water bound to collagen protein. A different imaging method, Ultrashort Echo Time (uTE) MRI, can image tissues with short T2. Prior studies have demonstrated UTE-T2* relaxation mapping of cartilage is sensitive to the short T2* component of cartilage 3 . Furthermore, bi-exponential relaxation models demonstrated better performance than mono-exponential models in articular cartilage 4 . This evidence supports the hypothesis that cartilage is composed of a short T2* component and a long T2* component 5, 6 , attributable to water bound to Proteoglycans/collagen fibers (T2 < 10 ms) and bulk free water7 , respectively.However, biexponential fitting to estimate the proportion of short and long T2 components requires multiple acquisitions and clinically unfeasible scan times. We propose a novel proxy for T2* based on the ratio of signal intensities at two different echo times which could reduce scan times while retaining similar information than T2*. Our work builds on the porosity index used successfully to estimate bone porosity8We calculate a ‘Free Water Index’ (FWI) using the ratio of image intensities from the signal collected at a ‘long’ echo time (above 1 ms) to the signal collected at the ‘ultra short’ echo time. The ultra short echo time signal will contain both the bound and free water components, while at the long echo time only the free water contributes to the signal, thus our ratio could potentially be a proxy measurement for free water in cartilage. The present study measures the correlation between the FWI and T2*.
Methods
Both knees of ten patients with clinically established OA and six healthy subjects were scanned using a 3T whole-body MRI scanner (GE healthcare Milqaukee, WI) with two 16-channel flexible phased-array coils (NeoCoil, Pewaukee WI). MR imaging included a double echo in steady state (DESS) sequence (TEs= 6.712 ms and 32.92 ms) for anatomical reference and a 5 echo ultrashort echo time (uTE) sequence (TEs = 0.032 ms, 3.4ms, 6.8ms, 10.2ms, and 13.6msArticular cartilage was automatically segmented and divided into 6 subregions (anterior, central and posterior for the medial and lateral side) using the open-source DOSMA framework on DESS scans 9. The uTE images were registered to the DESS scans using Elastix 10,11 . T2 maps were computed with DOSMA using an analytical signal model 12 . A monoexponential fit was used to obtain T2* maps and the FWI was computed according to eq. 1. To assess the optima choice for the long echo time, the FWI was evaluated using different long echo times
$$ \text{Free Water Index (%)} = \frac{\text{Echo}_{\text{Long}} {\text{Intensity}}} {\text{Echo}_{\text{Short}} {\text{Intensity}}} * 100 (1) $$
Pearson’s coefficient between T2*, T2, and the FWI was used to assess correlation.
Results
The average T2 for all subjects was 32.7 ms, T2* was 13.2 ms. The FWI for all subjects was 48.2%, 50.2%, 40.2%, and 34.5% using the 2nd, 3rd, 4th, and 5th echo, respectively. Each FWI showed a statistically significant correlation (P<0.001) with T2*. The Correlation was very strong (R2 > 0.9) using the FWI calculated with the 3rd, 4th, and 5th echo time and strong when using the second echo time (R2 >0.75). Results are detailed in Table 1. Correlation between FWI and T2* is shown in Figure 3 There were no significant within subject differences in T2, T2*, and the FWI between regions of cartilage. There was minimal correlation between T2 and T2* (R2 = .02, p = 0.50) for all knees collected.Visual inspection of the ratios show agreement between our ratio and T2* (Figure 5).
Discussion
Our results demonstrate in vivo feasibility of a novel ‘Free Water Index’, a potential proxy for UTE-T2*. UTE-T2* is an emerging measurement of early cartilage degradation, sensitive to changes in short T2* components such as water bound to collagen. The present study showed a high correlation between the FWI and T2* (R2 >0.9). Using our FWI, derived with 2 echoes, could shorten scan times while preserving the T2* signal. Although the work is exploratory, our FWI provides the basis for future work investigating optimal methods to shorten scan times while measuring cartilage degradation in vivo. Our work has a number of limitations. While T2* has been shown to be sensitive to changes in free water, the FWI has not been validated. Future studies should attempt to validate the FWI as a proxy for the proportion of bulk water in articular cartilage for both OA and healthy knees.Conclusion
The present work demonstrates in vivo feasibility of the Free Water Index, a proxy for uTE T2*. The FWI showed a high correlation to T2* and requires only 2 echoes, while T2* mapping often requires more than 4 echoes.Acknowledgements
This work was supported by GE Healthcare and NIH Grants R01 AR079431,U01 EB023829, and R01AR077604.References
- Chu, C. R., Millis, M. B., & Olson, S. A. (2014). Osteoarthritis: from palliation to prevention: AOA critical issues. The Journal of bone and joint surgery. American volume, 96(15).
- Bear, D. M., Szczodry, M., Kramer, S., Coyle, C. H., Smolinski, P., & Chu, C. R. (2010). Optical Coherence Tomography (OCT) Detection of Subclinical Traumatic Cartilage Injury. Journal of orthopaedic trauma, 24(9), 577.
- Williams, A., Qian, Y., Bear, D., & Chu, C. R. (2010). Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthritis and cartilage, 18(4), 539-546
- Sharafi, A., Chang, G., & Regatte, R. R. (2018). Biexponential T2 relaxation estimation of human knee cartilage in vivo at 3T. Journal of magnetic resonance imaging : JMRI, 47(3), 809–819.
- Shao, H., Chang, E. Y., Pauli, C., Zanganeh, S., Bae, W., Chung, C. B., Tang, G., & Du, J. (2016). UTE bi-component analysis of T2* relaxation in articular cartilage. Osteoarthritis and cartilage, 24(2), 364–373.
- Qian, Y., Williams, A. A., Chu, C. R., & Boada, F. E. (2010). Multicomponent T2* mapping of knee cartilage: technical feasibility ex vivo. Magnetic resonance in medicine, 64(5), 1426-1431.
- Zanganeh S. A., Shao H., Bydder G. M., Du J. (2017), Ultrashort Echo Time Imaging of Articular Cartilage. In Xia Y., Momot K. (1st Ed.), Biophysics and Biochemistry of Cartilage by NMR and MRI (pp. 299 - 319). Royal Society of Chemistry.
- Hong, A. L., Ispiryan, M., Padalkar, M. V., Jones, B. C., Batzdorf, A. S., Shetye, S. S., Pleshko, N., & Rajapakse, C. S. (2019). MRI-derived bone porosity index correlates to bone composition and mechanical stiffness. Bone reports, 11, 100213.
- Desai, A. D., Caliva, F., Iriondo, C., Mortazi, A., Jambawalikar, S., Bagci, U., ... & IWOAI Segmentation Challenge Writing Group. (2021). The international workshop on osteoarthritis imaging knee MRI segmentation challenge: a multi-institute evaluation and analysis framework on a standardized dataset. Radiology: Artificial Intelligence, 3(3), e200078.
- S. Klein, M. Staring, K. Murphy, M.A. Viergever, J.P.W. Pluim, "elastix: a toolbox for intensity based medical image registration," IEEE Transactions on Medical Imaging, vol. 29, no. 1, pp. 196 - 205, January 2010.
- D.P. Shamonin, E.E. Bron, B.P.F. Lelieveldt, M. Smits, S. Klein and M. Staring, "Fast Parallel Image Registration on CPU and GPU for Diagnostic Classification of Alzheimer's Disease", Frontiers in Neuroinformatics, vol. 7, no. 50, pp. 1-15, January 2014.
- Kogan, F., Levine, E., Chaudhari, A. S., Monu, U. D., Epperson, K., Oei, E., Gold, G. E., & Hargreaves, B. A. (2018). Simultaneous bilateral-knee MR imaging. Magnetic resonance in medicine, 80(2), 529–537.
Figures
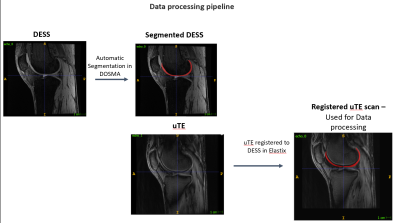
Figure
1: Processing pipeline to generate articular cartilage segmentation on uTE
scans
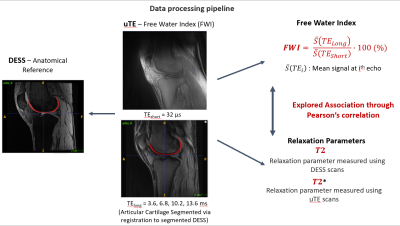
Figure 2: Data processing pipeline to
generate T2, T2*, and FWI.
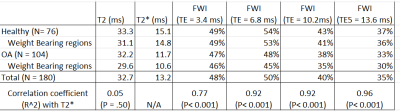
Table 1: Outlines average values for T2,
T2* and the FWI calculated with each echo time. The table also shows the
correlation coefficient between the each measurement and T2*.
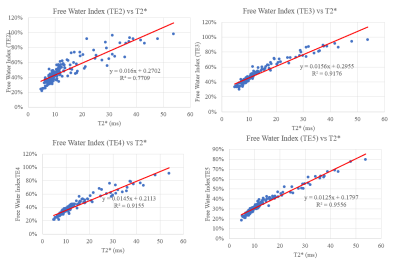
Figure 3: Correlation between T2* and the
FWI for all subjects.
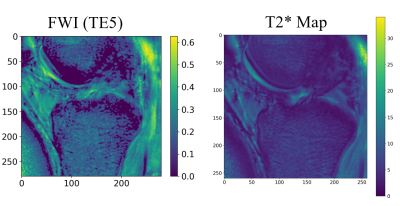
Figure 4: FWI calculated using the fifth
echo compared to T2* map. Visual inspection shows a high level of agreement of
each map.
DOI: https://doi.org/10.58530/2023/1635