1633
T2 Mapping with GRAPPATINI for Routine Knee Evaluation1Department of Radiology and Imaging, Hospital for Special Surgery, New York, NY, United States, 2Weill Cornell Medicine, New York, NY, United States, 3Siemens Medical Solutions USA, Inc., New York, NY, United States, 4Advanced Clinical Imaging Technology, Siemens Healthineers International AG, Lausanne, Switzerland, 5Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 6LTS5, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Synopsis
Keywords: Quantitative Imaging, Parallel Imaging, T2 mapping, GRAPPATINI
T2 mapping with GRAPPATINI, a combination of MARTINI (model-based reduction) and GRAPPA (parallel imaging reconstruction), was applied to routine knee evaluations and compared quantitatively to standard T2 mapping accelerated only with GRAPPA. Regions of articular cartilage, infrapatellar fat pad, and muscle were analyzed. T2 mapping with GRAPPATINI was faster by ~2.2x and provided similar T2 values in most regions except for the infrapatellar fat pad and femoral trochlea cartilage. This accelerated T2 mapping technique may facilitate the routine incorporation of quantitative T2 mapping retrospective knee studies of osteoarthritis in the cartilage and of muscle denervation.Introduction
Anatomic structures in the knee, including articular cartilage,1 the infrapatellar fat pad (IPFP), and muscle are affected by degenerative changes or pathologies such as osteoarthritis, inflammation,2 or denervation.3 The quantitative MRI (qMRI) technique of T2 mapping is complementary to standard morphologic imaging and provides a means to evaluate the biochemical composition of imaged tissues. However, T2 mapping is not routinely used in clinical practice, in part because of the long scan time (>6 minutes) required to achieve spatial resolution adequate for tissue analysis of small or thin structures. Data undersampling in k-space with parallel imaging methods such as GRAPPA can improve scan times.4 Additional undersampling by employing a model-based reconstruction with GRAPPATINI can also provide further acceleration.5 While GRAPPATINI may be used to provide synthetic fat-saturated knee images for clinical assessment,6 T2 maps are less commonly acquired with fat saturation and can bias quantitation. In this preliminary study, we compared T2 values from the GRAPPATINI acquisition to T2 values from a standard multi-echo spin echo (MESE) acquisition, both without fat suppression, during routine knee evaluations. We hypothesized that quantitative T2 values of articular cartilage, IPFP, and muscles would be comparable to those from standard MESE T2 mapping. We also hypothesized that there would be consistency of GRAPPATINI and MESE T2 values between scanners as determined from phantom scanning.Methods
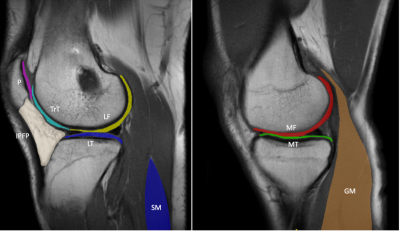
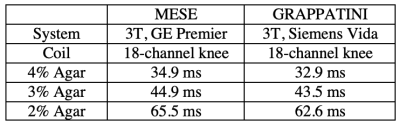
9 patients (4F/5M, mean age = 45, range = 27 - 72 years) undergoing routine knee evaluations on a clinical 3-Tesla MRI (MAGNETOM Vida, Siemens Healthcare, Erlangen, Germany) provided written consent for this IRB-approved study. A MESE T2 mapping sequence accelerated with GRAPPA (MapIt sequence, FOV = 18cm, matrix = 336 x 236, 5 mm slice (1 mm gap), 14 slices, TR = 1600 msec, first and delta TE = 10.2 msec, 8 echoes, BW = 354 Hz/pixel, GRAPPA = 2, nominal scan time = 4:10 min) was acquired using an 18-channel knee coil. A GRAPPATINI research application sequence was also acquired (TR=2020 msec, 10 echoes, GRAPPA = 2, model-based acceleration = 3, scan time = 1:52 min, Fig. 1). Apparent proton density-weighted images (TE = 0 ms) and synthetic T2-weighted images (TE = 80 ms) were also obtained from GRAPPATINI. This provided a total of n=9 paired T2 maps for comparison. For analysis, image segmentation was performed using ITK-SNAP7 in 9 regions of interest (ROI) and nominally on 3 slices per ROI: medial femoral condyle cartilage (MF), medial tibia cartilage (MT), lateral tibia cartilage (LT), lateral femoral condyle cartilage (LF), femoral trochlea cartilage (TrT), patella cartilage (P), infrapatellar fat pad (IPFP), soleus muscle (SM), and the medial head of the gastrocnemius muscle (GM) (Fig. 2). Paired comparison of the T2 values was performed using Wilcoxon signed-rank tests where p<0.05 was deemed statistically significant. Linear regression was also performed across all regions of GRAPPATINI against MESE. To compare the consistency of GRAPPATINI T2-values between scanners, a phantom with varying T2 values (35 to 65 msec) was scanned using GRAPPATINI and with a standard MESE sequence using an 18-channel knee coil on a separate clinical 3-Tesla MRI scanner (GE Healthcare).Results
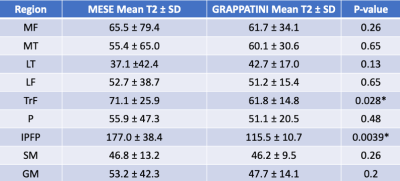
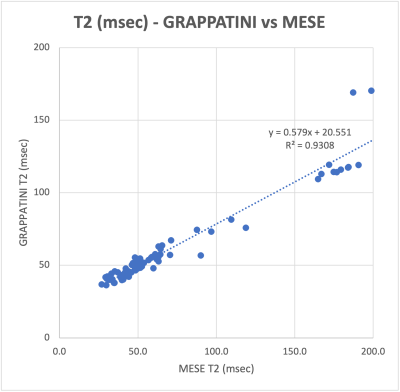
In patients, the T2 values of most ROIs did not differ significantly between the MESE and GRAPPATINNI imaging acquisitions (Table 1), except for the IPFP (T2MESE = 177.0 ± 38.4 msec, T2GRAPPATINI = 115.5 ± 10.7 msec, p = 0.0039) and TrT (T2MESE = 71.1 ± 25.9 msec, T2GRAPPATINI = 61.8 ± 14.8 msec, p = 0.028) regions. Overall, the T2 values between GRAPPATINI and MESE were linearly related, with an R2 of 0.931 (Fig. 3). The phantom comparison showed similar T2 values between GRAPPATINI and standard MESE sequences on different 3-Tesla MRI scanners (Table 2).Discussion
Quantitative comparisons found that GRAPPATINI T2 mapping allowed for faster exams with reasonable agreement compared to standard MESE T2 mapping. T2 mapping with GRAPPATINI saved ~2.2x of the scan time despite using a longer TR to allow for T1 recovery. This may facilitate faster routine knee evaluations of cartilage and muscle. The differences observed in the IPFP and TrT require further investigation. These might be due to differences in T2-fitting techniques in MESE and GRAPPATINI, as GRAPPATINI ignored the first echo to mitigate the effects of stimulated echoes caused by imperfect refocusing pulses and B1 inhomogeneity.Conclusion
T2 mapping with GRAPPATINI allows for faster acquisition times than standard MESE T2 mapping while producing similar T2 values. The results of this study highlights the potential of T2 mapping with GRAPPATINI in routine clinical use.Acknowledgements
HSS receives institutional research support from Siemens Medical Solutions USA, Inc.References
1. Friedrich, K. M. et al. T2 Measurements of Cartilage in Osteoarthritis Patients With Meniscal Tears. Am J Roentgenol 193, W411–W415 (2009).
2. Sacher, S. E. et al. MAVRIC based T2 mapping assessment of infrapatellar fat pad scarring in patients with total knee arthroplasty. J Orthop Res (2022) doi:10.1002/jor.25472.
3. Argentieri, E. C. et al. Quantitative T2‐mapping magnetic resonance imaging for assessment of muscle motor unit recruitment patterns. Muscle Nerve 63, 703–709 (2021).
4. Griswold, M. A. et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnet Reson Med 47, 1202–10 (2002).
5. Hilbert, T. et al. Accelerated T2 mapping combining parallel MRI and model‐based reconstruction: GRAPPATINI. J Magn Reson Imaging 48, 359–368 (2018).
6. Roux, M. et al. MRI T2 Mapping of the Knee Providing Synthetic Morphologic Images: Comparison to Conventional Turbo Spin-Echo MRI. Radiology 182843 (2019) doi:10.1148/radiol.2019182843.7. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. PMID: 16545965.
Figures