1631
Highly efficient simultaneous joint T1-T2 mapping for isotropic resolution 3D knee imaging1Millennium Institute for Intelligent Healthcare Engineering (iHEALTH), Santiago, Chile, 2Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 3King's College London, London, United Kingdom, 4Electrical Engineering Department, Pontificia Universidad Católica de Chile, Santiago, Chile, Santiago, Chile, 5ShanghaiTech University, Shanghai, China
Synopsis
Keywords: Quantitative Imaging, Osteoarthritis
Recently, a free-running T1-T2 3D radial imaging sequence was proposed to enable simultaneous acquisition of T1 and T2 maps of the hearth with isotropic spatial resolution. Here, we sought to investigate the feasibility of this sequence with 1 mm3 isotropic spatial resolution for the characterization of articular cartilage in scan time of ~4 min. The proposed approach was evaluated on a standardized phantom and in-vivo in healthy subjects, showing good agreement with reference values.
Introduction
Osteoarthritis (OA) is a multi-systemic degenerative disease characterised by structural and biochemical deterioration of the hyaline articular cartilage 1. It is also one of the most common and disabling joint diseases 1. Quantitative MRI techniques such as T1 and T2 mapping provide relevant information on macromolecular changes in cartilage and are a promising effective and non-invasive approach to identify early cartilage degeneration 2. However, conventional T1 and T2 mapping techniques require long acquisition times and are usually performed sequentially in separate 2D scans 3. Furthermore, most approaches are limited to anisotropic resolution to reduce scan time. The aim of this study is to determine the feasibility of a recently proposed free-running T1- T2 mapping 3D radial sequence 4 to enable multiparametric characterization of articular cartilage with isotropic spatial resolution of 1mm3 in ~4 min scan time.Methods
The proposed knee joint T1-T2 mapping sequence consists of water-selective spoiled gradient echo readout with a 3D golden-angle radial trajectory 4. Each shot interval is preceded by an inversion recovery (IR) pulse and a T2 preparation pulse (T2-prep) with varying echo times of 0 ms (no T2-prep), 30 ms and 60 ms, respectively. This acquisition pattern is repeated to provide enough T1 and T2 encoding (Figure 1).The proposed approach was evaluated on a standardized T1MES phantom 8 and in-vivo experiments in a healthy subject. Acquisitions were performed on a 3T scanner (Philips, Ingenia) with a 16-channel knee coil. Main acquisition parameters include: FOV= 120 mm, isotropic resolution= 1 mm, flip angle = 6°, TR/TE=10.3 ms/ 4.1ms, acquisition pattern repeated 100 times with 195 readouts in each shot, IR repetition time = 2200 ms, total scan time = 4.5 min.
After the acquisition data was binned into 10 different IR times for eachT2-prep. The reconstruction of 5 compressed contrasts 5 was performed using dictionary based low rank inversion with high-dimensional patch-based low-rank regularization (HD-PROST) 6. The dictionary D, which provides the signal evolution for different combinations of T1 and T2 values, was generated using Bloch simulations. Values for T1 were between 200 ms and 3000 ms with increments of 2%, and values for T2 were between 10 ms and 250 ms with increments of 1%.
Images were recovered by minimizing the following Lagrangian with ADMM:
$$L(x,T, Y)=∥Ex−Wk∥^2_2+λ∑_p∥T_p∥_∗+μ/2∑_p∥T_p−Pp(x)−P_p(Y)∥^2_F$$
Where $$$x∈C^{(Nr×1)}$$$ is the multi-contrast complex image with $$$N$$$ voxels and $$$r$$$ different singular images corresponding to the first $$$r$$$ singular values of the dictionary compression, $$$k∈C^{KN_c×1}$$$ is the k-space acquired data with $$$K$$$ samples and $$$N_c$$$ coils, $$$W$$$ is a k-space density compensation matrix, $$$E=WFU_rS$$$ is the encoding operator with $$$S∈C^{NN_cr×Nr}$$$ the estimated sensitivity maps, $$$F∈C^{KN_c×NN_cL}$$$ the non-uniform Fourier transform operator with $$$L$$$ the total number of contrasts, and $$$U_r∈R^{NN_cL×NN_cr}$$$ the compressed signal-evolution dictionary with the highest $$$r$$$ singular values of the dictionary compression. $$$T_p$$$ is the HD-PROST tensor formed with similar patches centered at voxel $$$p$$$, $$$P_p(∙)$$$ is the patch-selecting operator centred at voxel $$$p$$$, $$$Y$$$ are the augmented Lagrange multipliers, $$$λ$$$ and $$$μ$$$ are regularization parameters, and $$$∥∙∥_*$$$ , $$$∥∙∥_F$$$ are the nuclear and Frobenius norms. Main reconstruction parameters were set to $$$r=5$$$, $$$λ=0.04$$$.
After obtaining $$$x$$$, a phase-sensitive dictionary matching was used to obtain T1 and T2 maps. Transverse magnetization sign was recovered for each complex-image 7 and a real image $$$x_s$$$ was obtained. Dot-product matching was performed between the previously generated dictionary $$$D$$$ and $$$x_s$$$ to estimate the maps.
Results
Phantom: Proposed joint T1 and T2 maps for the standardized phantom are shown in Figure 2. Correlation plots between the T1 and T2 values obtained for each vial with the proposed approach and reference values provided by the vendor. T1 and T2 values measured with the proposed approach are in very good agreement with reference values for the range of interest, with high values (higher than ~1500 ms for T1 and higher than ~150 ms for T2) being over-estimated for T1 and under-estimated for T2, because of the choice of echo times for -prep and IR time.Healthy subject: Proposed joint T1 and T2 maps for a representative healthy subject is shown in Figure 3 in all three orthogonal dimensions. The T1 and T2 values of the hyaline cartilage of the knee using the proposed simultaneous free-running T1-T2 sequence were T1 = 898.99 ± 83.73 ms and T2= 22.91 ± 3.79 ms, which are in agreement with clinical values reported in the literature 2,3 which corresponds to T1=907.42 ± 140.53 ms and T2 = 34.74±2.48 ms.
Discussion and conclusion
In this study we demonstrate the feasibility of simultaneous joint T1-T2 mapping of the knee with a free-running 3D radial sequence for quantitative evaluation of hyaline articular cartilage with isotropic spatial resolution of 1 mm3 in ~4 min total scan time. The proposed approach achieves T1 and T2 values comparable to reference values reported in the literature. Further studies will compare the proposed approach against conventional mapping in healthy subjects and patients with osteoarthritis.Acknowledgements
This work was funded by ANID – Millennium Science Initiative Program – ICN2021_004.
References
1. Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016 Dec;59(5-6):333-339.
2. Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S. In vivo T(1rho) and T (2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007 Jul;15(7):789-97.
3. Mittal S, Pradhan G, Singh S, Batra R. T1 and T2 mapping of articular cartilage and menisci in early osteoarthritis of the knee using 3-Tesla magnetic resonance imaging. Pol J Radiol. 2019 Dec 18;84: e549-e564.
4. Qi H, Bustin A, Cruz G, Jaubert O, Chen H, Botnar RM, Prieto C. Free-running simultaneous myocardial T1/T2 mapping and cine imaging with 3D whole-heart coverage and isotropic spatial resolution. Magn Reson Imaging. 2019 Nov; 63:159-169.
5. McGivney DF, Pierre E, Ma D, Jiang Y, Saybasili H, Gulani V, Griswold MA. SVD compression for magnetic resonance fingerprinting in the time domain. IEEE Trans Med Imaging. 2014 Dec;33(12):2311-22.
6. Bustin A, Lima da Cruz G, Jaubert O, Lopez K, Botnar RM, Prieto C. High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI. Magn Reson Med. 2019 Jun;81(6):3705-3719.
7. Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002 Feb;47(2):372-83.
8. Captur G, Gatehouse P, Keenan KE, Heslinga FG, Bruehl R, Prothmann M, Graves MJ, Eames RJ, Torlasco C, Benedetti G, Donovan J, Ittermann B, Boubertakh R, Bathgate A, Royet C, Pang W, Nezafat R, Salerno M, Kellman P, Moon JC. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance-the T1 Mapping and ECV Standardization in cardiovascular magnetic resonance (T1MES) program. J Cardiovasc Magn Reson. 2016 Sep 22;18(1):58. doi: 10.1186/s12968-016-0280-z. PMID: 27660042; PMCID: PMC5034411.
Figures
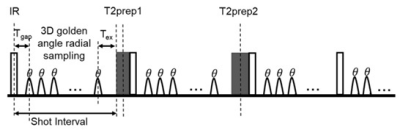
Fig 1. Proposed 3D knee joint T1- T2 mapping using 3D radial golden angle trajectory 4. Each shot interval is preceded by an inversion recovery (IR) pulse and a T2 preparation pulse (T2-prep) with varying echo times of 0ms (no T2-prep), 30 ms (T2-prep1) and 60 ms (T2-prep2), respectively. This acquisition pattern is repeated several times to provide T1and T2 encoding.
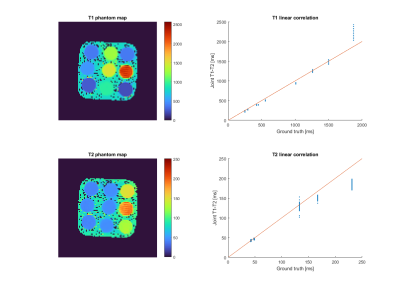
Fig 2. Proposed joint T1 and T2 maps for the standardized T1MES phantom (left). Correlation plots between the proposed joint T1-T2 values and reference values provided by the vendor are also included (right).

Fig 3. (a) Axial view, (b) Sagittal view and (c) Coronal view of knee in a healthy subject: i) Identification of regions of interest (ROI) used for quantification in a single contrast image (red arrows) . ii) T1 map. iii) T2 map.