1627
Quantitative Parameter Mapping in the Abdomen at 7T using Radial QTI Encoding1GE Healthcare, Munich, Germany, 2IRCCS Stella Maris, Pisa, Italy, 3THI, Ingolstadt, Germany, 4Imago7, Pisa, Italy
Synopsis
Keywords: MR Fingerprinting/Synthetic MR, MR Fingerprinting
Quantitative MRI offers diagnostic insights into tissue properties and enables the characterisation of diseases, while reducing variability between operators, sites and vendors. A 2D and a 3D radial sequence using Quantitative Transient State Imaging (QTI) were implemented and optimised to map T1, T2 and Proton Density in the abdomen at 7T. Resulting in-vivo multi-parametric maps were of motivating quality, while validation in the Eurospin TO5 phantom showed good agreement with T1 and T2 Gold standard measurements.Introduction
Quantitative MRI is a way to provide reproducible data across different operators, sites and vendors. Quantitative Transient State Imaging (QTI) [1], which shares some concepts with MR Fingerprinting [2], offers fast and simultaneous mapping of T1, T2 relaxation times and Proton Density (PD) with high reproducibility and repeatability [3]. Most studies and developments focused on lower (1.5T and 3T) field strengths and on brain. Higher field strengths, such as 7T, are more challenging because B0 inhomogeneities and chemical shifts scale with B0, while dielectric effects significantly reduce transmit B1+ homogeneity. QTI in abdominal regions is considerably more challenging even at 3T because of exacerbated B0 homogeneities and motion artefacts as compared to QTI in the brain [4]. The goal of this work was to implement a 2D and a 3D radial QTI sequence and to optimise it for parameter mapping in the abdomen at 7T.Methods
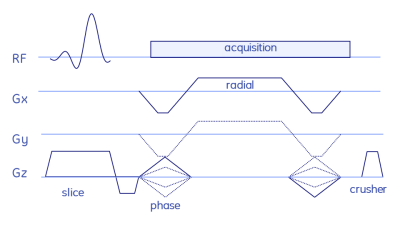
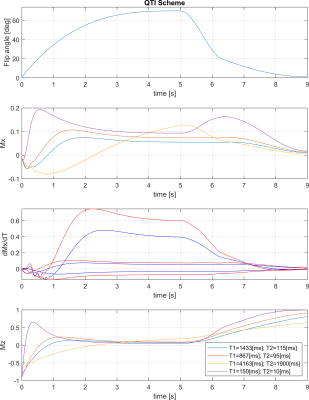
For the 2D radial sequence, the full-spoke radial trajectory was rotated in 2D space (logical xy) via golden angles. For the 3D stack-of-stars version Cartesian phase encoding blips were added to encode the third dimension (logical z) (Fig. 1). The QTI flip angle scheme and its optimisation are depicted and explained in Fig. 2. Radial QTI encoding was designed in Matlab and executed on the MRI scanner via the MNS Research Pack, a flexible multi-release sequence environment to read in gradient waveforms, RF pulses and parameters such as flip angle modulations from files. Two volunteers were scanned in the abdomen on a 7T MRI whole-body scanner (GE Healthcare) using an 8-channel dipolar transmit-receive coil assembly (Tesla Dynamic Coils). Five gel-filled vials of the Eurospin TO5 phantom with different T1 and T2 were scanned with a 32-channel receive head coil (Nova Medical). Sequence parameters are listed in Figs. 3 and 4. One QTI train consists of one inversion pulse upfront and 1000 excitation with varying flip angles (Fig. 2).Data was reconstructed via SVD-compression, gridding, coil combination, apodisation and matching pursuit to simulated Extended Phase Graphs.
Results and Discussion
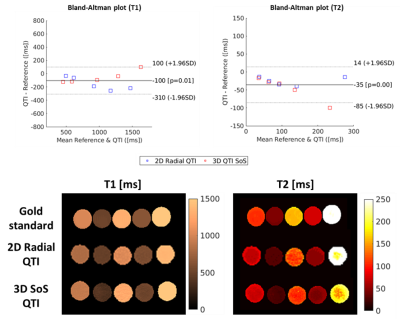
Representative axial and coronal slices of the obtained T1, T2 and PD maps of the abdomen for 2D and 3D Radial QTI are shown in Figs. 3 and 4, respectively. T1 and T2 times agree well with Gold standard comparison measurements at 7T (Fig. 5): T1/T2 Intraclass Correlation Coefficients (ICCs) were 0.95 / 0.98 for 2D radial QTI and 0.99 / 0.89 for 3D stack-of-stars QTI, respectively. Compared to parameter mapping at 3T in the brain, QTI in the abdomen is far more challenging due to B0 and B1 inhomogeneities, and because of motion. Considering these challenges, obtained image qualities cannot compete with results at lower field strengths, but still represent a step forward towards rapid quantitative parameter mapping in the abdomen at higher field strengths. Combining Radial QTI with parallel transmit excitation should considerably improve B1+ homogeneities, hence reduce the signal dropout in the abdomen.Image reconstruction and parameter inference (including DICOM export) is implemented on the MRI scanner to facilitate a clinical workflow. Currently, reconstruction times were in the same order of magnitude as the acquisition time and could be accelerated by using newer computers or GPUs.
Conclusion
MR multi-parameter mapping using the Radial QTI sequence yields reasonable quality T1, T2 and PD maps of the abdomen in humans in reasonable scan times.Acknowledgements
EU H2020 CHAIMELEON grant (#952172), EU FET NICI grant (#801075).References
[1] Rapid three-dimensional multiparametric MRI with quantitative transient-state imaging. Gómez PA, Cencini M, Golbabaee M, Schulte RF, Pirkl C, Horvath I, Fallo G, Peretti L, Tosetti M, Menze BH, Buonincontri G. Sci Rep. 2020 Aug 13;10(1):13769. doi: 10.1038/s41598-020-70789-2.
[2] Magnetic resonance fingerprinting. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Nature. 2013 Mar 14;495(7440):187-92. doi: 10.1038/nature11971.
[3] Three dimensional MRF obtains highly repeatable and reproducible multi-parametric estimations in the healthy human brain at 1.5T and 3T. Buonincontri G, Kurzawski JW, Kaggie JD, Matys T, Gallagher FA, Cencini M, Donatelli G, Cecchi P, Cosottini M, Martini N, Frijia F, Montanaro D, Gómez PA, Schulte RF, Retico A, Tosetti M. Neuroimage. 2021 Feb 1;226:117573. doi: 10.1016/j.neuroimage.2020.117573.
[4] Quantitative Parameter Mapping Of Prostate Using Stack-Of-Stars And QTI Encoding. Schulte RF, Pirkl CM, Garcia-Polo P, Cencini M, Tosetti M, Marti-Bonmati L, Menzel MI. ISMRM 2022, #0182.
[5] Optimal experiment design for magnetic resonance fingerprinting. Bo Zhao, Haldar JP, Setsompop K, Wald LL. Annu Int Conf IEEE Eng Med Biol Soc. 2016 Aug;2016:453-456. doi: 10.1109/EMBC.2016.7590737.
Figures