1624
3D whole-heart free-breathing joint T1/T2 mapping with isotropic resolution in a hybrid PET-MR system for cardiac sarcoidosis1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Camberley, United Kingdom, 3Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
Keywords: Quantitative Imaging, Cardiovascular
Simultaneously acquired 18F-FDG PET-MR imaging and quantitative 2D T1 and T2 mapping have been suggested for improved diagnostic accuracy of cardiac sarcoidosis, however misregistration between imaging modalities and sequential MR scans makes clinical interpretation challenging. Here we evaluate the feasibility of recently proposed 3D joint T1/T2 sequence at a 3T PET-MR system. This approach enables non-rigid motion-correction for both the 3D T1/T2 mapping and the PET data to the same respiratory position, resulting in aligned volumes for improved clinical interpretation. In this proof-of-concept study, we tested our approach in a phantom and two healthy subjects.Introduction
Clinical guidelines for the diagnosis of cardiac sarcoidosis (CS) currently suggest a combined approach using a number of different investigations, including late gadolinium enhancement (LGE) cardiac MR (CMR) and 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) in addition to biopsy1. For improved diagnosis, simultaneously acquired 18F-FDG PET-MR imaging and quantitative T1 and T2 mapping2,3 have been suggested, however, respiratory motion related mis-registration between conventional cardiac MR data, usually acquired in 2D under multiple breath holds, and simultaneously acquired 3D free-breathing PET data remains a challenge when interpreting imaging findings, thus hindering clinical adoption. Moreover, respiratory motion degrades both image quality and quantification of PET and MR images. To overcome these challenges, we propose a highly efficient, motion-compensated comprehensive PET-MR framework for cardiac sarcoidosis, providing myocardial tissue characterization from novel 3D joint T1/T2 MR parametric maps as well as non-rigid respiratory motion fields to correct simultaneously acquired PET data4 in a 3T hybrid PET-MR system. In this proof-of-concept study, we evaluate the proposed joint T1/T2 mapping sequence in a phantom and two healthy subjects.Methods
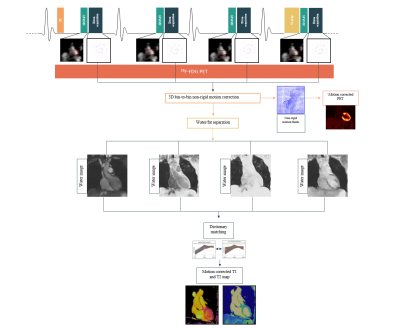
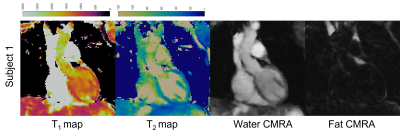
Acquisition & Reconstruction: An ECG-triggered free-breathing 3D whole-heart joint T1/T2 research sequence was implemented on a 3T PET-MR system (Biograph mMR, Siemens Healthcare, Erlangen, Germany) (Figure 1). Four interleaved volumes are acquired using two point Dixon GRE readout and a 4-fold undersampled variable-density Cartesian trajectory5. The sequence includes four interleaves with 1) Inversion Recovery (IR) preparation, 2) and 3) no-preparation, 4) T2-preparation (T2prep) respectively6. Image navigators (iNAVs)7 are integrated in the sequence to enable 100% respiratory scan efficiency and predictable scan time. 3D non-rigid motion is estimated and incorporated into a motion-compensated reconstruction8 with patch-based low-rank regularization9 to produce motion-compensated datasets. A water-fat separation algorithm10 is used to generate water and fat images for each dataset, and the water images are used to obtain the signal evolution across the acquired volumes. T1 and T2 maps are then computed using dictionary-matching with a dictionary generated using an EPG simulation11.Imaging & Analysis: A phantom and two healthy subjects were scanned on a 3T PET-MR scanner using the proposed approach. Data was acquired during mid-diastole (acquisition window = 92±15ms). MR imaging parameters included: coronal orientation, FOV=320x320x120mm3, 2mm3 isotropic resolution, T2 prep=40ms, TI=250ms, TE1/TE2/TR=2.30/3.75/5.42ms, flip angle=15°, acquisition time ~ 6min. Conventional breath-held 2D T1 and T2 maps were also acquired in short axis orientation at apical, mid-cavity and basal slices for comparison purposes (imaging parameters 2D T2 mapping: resolution = 1.5x1.5x8mm3, T2-preparation pulses = 0, 28, 55 ms, flip angle=12°; imaging parameters 2D T1 mapping: resolution = 1.4x1.4x8mm3, TI = 100ms, flip angle=35°). ROI was selected at the septum, where mean and standard deviation were calculated for both subjects.
Results
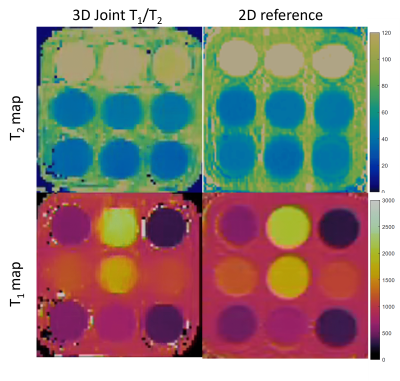
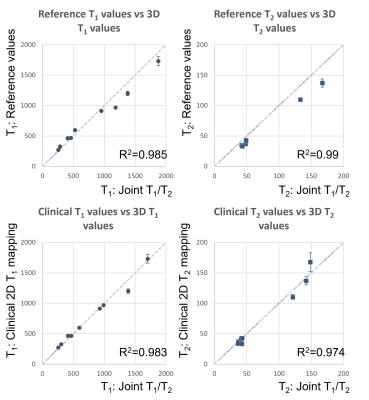
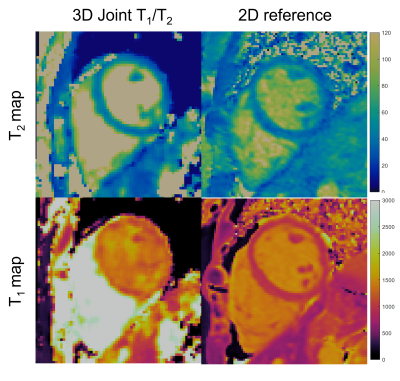
Joint T1/T2 maps of the phantom in comparison to conventional maps acquired clinically and reference values from12 are shown in Figure 2 and Figure 3, where comparable values between the proposed technique and the conventional method and reference values for both T1 (R2=0.983, R2=0.985) and T2 maps (R2=0.974, R2=0.99) are observed. T1 and T2 maps alongside water and fat images for both healthy volunteers are shown in Figure 4. T1 and T2 maps from the proposed joint T1/T2 approach are shown in Figure 5, along with the conventional 2D T1 and 2D T2 reference maps obtained during the same examination for a healthy subject. Values for 3D approach were: T1=1330.7286ms, T2=38.9447ms, compared to values obtained with the 2D approach: T1=1315.1429, T2=39.26ms.Conclusion
Preliminary results of the proposed joint T1/T2 mapping at 3T hybrid PET-MR system, that allows acquisition of isotropic T1 and T2 maps and complimentary co-registered water/fat anatomical datasets, demonstrate the feasibility of this technique. Further studies in a larger cohort of healthy subjects and patients with cardiac sarcoidosis undergoing a PET/MR scan are warranted.Acknowledgements
This work was supported by the following grants: (1) EP/L015226/1, EPSRC P/V044087/1; (2) BHF programme grant RG/20/1/34802, (3) Wellcome/EPSRC Centre for Medical Engineering (WT 203148/Z/16/Z), (4) Millennium Institute for Intelligent Healthcare Engineering ICN2021_004, (5) FONDECYT 1210637 and 1210638, (6) IMPACT, Center of Interventional Medicine for Precision and Advanced Cellular Therapy, ANID FB210024.References
1. Birnie, D. H. et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Hear. Rhythm 11, 1305–23 (2014).
2. Crouser, E. D., Ono, C., Tran, T., He, X. & Raman, S. V. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am. J. Respir. Crit. Care Med. 189, 109–12 (2014).
3. Cheung, E. et al. Combined simultaneous FDG-PET/MRI with T1 and T2 mapping as an imaging biomarker for the diagnosis and prognosis of suspected cardiac sarcoidosis. Eur. J. hybrid imaging 5, 24 (2021).
4. Munoz, C. et al. Motion‐corrected simultaneous cardiac positron emission tomography and coronary MR angiography with high acquisition efficiency. Magn. Reson. Med. 79, 339 (2018).
5. Prieto, C. et al. Highly efficient respiratory motion compensated free-breathing coronary mra using golden-step Cartesian acquisition. J. Magn. Reson. Imaging 41, 738–746 (2015).
6. Milotta, G. et al. 3D whole-heart isotropic-resolution motion-compensated joint T(1) /T(2) mapping and water/fat imaging. Magn. Reson. Med. 84, 3009–3026 (2020).
7. Henningsson, M. et al. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn. Reson. Med. 67, 437–445 (2012).
8. Batchelor, P. G. et al. Matrix description of general motion correction applied to multishot images. Magn. Reson. Med. 54, 1273–1280 (2005).
9. Bustin, A. et al. Accelerated free-breathing whole-heart 3D T2 mapping with high isotropic resolution. Magn. Reson. Med. 83, 988–1002 (2019).
10. Liu, J., Peters, D. C. & Drangova, M. Method of B0 mapping with magnitude-based correction for bipolar two-point Dixon cardiac MRI. Magn. Reson. Med. 78, 1862–1869 (2017).
11. Weigel, M. Extended phase graphs: Dephasing, RF pulses, and echoes - pure and simple. J. Magn. Reson. Imaging 41, 266–295 (2015).
12. Captur, G. et al. A medical
device-grade T1 and ECV phantom for global T1 mapping quality assurance—the T1
Mapping and ECV Standardization in cardiovascular magnetic resonance (T1MES)
program. J. Cardiovasc. Magn. Reson. 18, 58 (2016).
Figures