1607
Insensitive to Off-Resonance 3D T1/T2 Mapping SPGR/bSSFP using Dictionary-Matching: Application to the Prostate at 3T1Biomedical Imaging Center-Universidad Catolica de Chile, Santiago, Chile, 2Millenium Institute for Intelligent Healthcare Engineering, Santiago, Chile, 3Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, Santiago, Chile, 4King's College London, London, United Kingdom, 5Departamento de Radiologia-Universidad Católica de Chile, Santiago, Chile, 6Department of Electrical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
Keywords: Quantitative Imaging, Prostate
Balanced Steady State Free Precession (bSSFP) has been successfully used to estimate T2 maps (DESPOT2), but bSSFP is prone to off-resonance artifacts. These artifacts affect the reconstruction of the T2 maps. We propose a dictionary-matching reconstruction using the signal model of bSSFP and spoiled gradient echo (SPGR). We tested this approach with simulation, 3D phantom and 3D in-vivo prostate acquisitions. In all of them, the off-resonance banding artifacts are greatly reduced, which is reflected in reduced NRMSE values. This method allows using a faster sequence with high SNR to quantify complicated body regions like the prostate.Introduction
Spoiled gradient echo (SPGR) and balanced steady-state free precession (bSSFP) have been successfully used by DESPOT to simultaneously estimate T1 and T2 values in the 3D brain1. The advantages of using bSSFP are its high SNR and scan efficiency. However, banding artifacts produced by off-resonance affect the quality of the parametric maps2. The severity of the banding artifact is proportional to the repetition time, which motivates shorter TRs at the cost of spatial resolution2. Even with short TRs there are areas with high magnetic susceptibility that still produce artifacts (sinuses, prostate area, and others). We propose to use a dictionary-matching of fingerprints constructed using the signal model of bSSFP and SPGR sequences in the spatial domain. The feasibility of the proposed approach was evaluated in simulations, 3D standardized T1/T2 phantom, and in-vivo acquisitions.Method
T1 and T2 map reconstructions:
- Dictionary approach: each entry of the dictionary was obtained using the following equations:
where $$$a = -(1-E_{1})E_{2}sin(\alpha) $$$, $$$(1-E_{1})sin(\alpha) $$$, $$$ d = E_{2}(E_{1}-1)(1+cos(\alpha))$$$, $$$c = 1 - E_{1}cos(\alpha)-(E_{1}-cos(\alpha))E_{2}^{2} $$$, $$$ E_{1,2}=e^{-\frac{TR}{T_{1,2}}}$$$, $$$ \Delta f$$$ is the off-resonance. And, for SPGR the equation is:
$$ m_{SPGR} = \frac{1-E_{1}}{1-cos(\alpha)E_{1}}sin(\alpha)e^{-\frac{TE}{TR}}$$
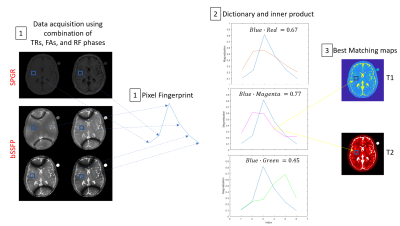
The range of T1, T2 and $$$\Delta f$$$ values: T1(200 to 2500 ms, every 10 ms), T2 (10 to 300 ms, every 4 ms), and $$$\Delta f $$$ (-450 to 450 Hz, every 4 Hz). Parametric values were obtained by estimating the measured signal to the closest dictionary entry using the maximum dot product (see Fig. 1).
- Comparison method: we compared our reconstruction with DESPOT1 and DESPOT2 (using the method of Deoni et al. 20031). As a quality measurement, we used the Normalised Mean Square Error for T1 and T2.
bSSFP and SPGR acquisitions: our method (dictionary approach) was tested in simulations, phantom and in-vivo prostate acquisitions. The acquisitions were done in a 3T Achieva MR system (Philips, Best, The Netherlands). Two 3D SPGR acquisitions were done with Flip Angles, α = 4º and 15º (TR/TE = 9.0/3.46 ms). Additionally, three 3D bSSFP acquisitions with α (phase) = 15° (180°), 45° (180°) and 60° (180°), with TRs = 3.6, 4.8, and 6.2 ms. The FOV was 128x128 mm2 (simulations) and 160x160x108 mm3 (phantom and in-vivo acquisitions) with a resolution of 1x1x3 mm3.
Results
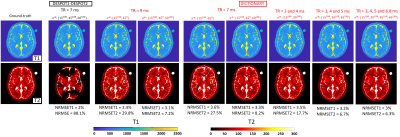
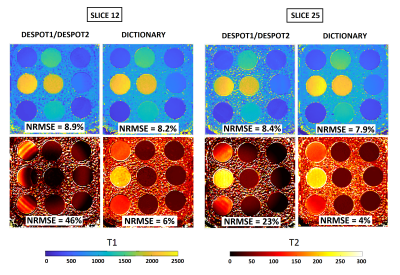
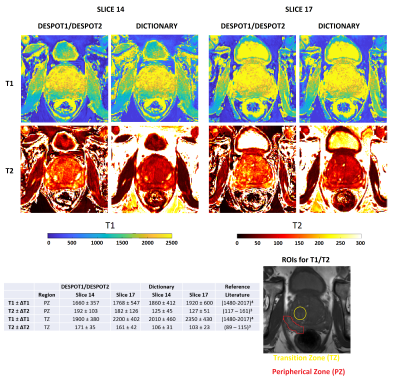
Figure 2 shows simulations using classical DESPOT1/DESPOT2 and Dictionary. DESPOT2 presents an important loss of signal with banding artifacts. On the contrary, our approach using Dictionary improve considerably the reconstruction for all TR, α (flip angle), and φ (RF phase) combination. The best results are when φ is alternated between 0 and 180 degrees, and when φ is fixed but TR varies. Both combinations move the bands between the contrast images decreasing the off-resonance effect. Thus, the NRMSE is reduced from 88% (DESPOT2) to around 7% (Dictionary). Figure 3 shows the reconstructed T1 and T2 maps using DESPOT1/DESPOT2 and the proposed dictionary approach for a 3D phantom. The reference ground truths for the phantoms were the values provided by the vendor5. Banding artifacts and loss of signal in T2 maps are clearly decreased using dictionary in comparison to DESPOT2. The T2 NRMSEs were reduced from 46%/26% (DESPOT2, slices 12/25) to 6%/4% (Dictionary, slices 12/25). T1 maps are similar for both methods. Figure 4 shows in-vivo reconstructed 3D prostate T1 and T2 maps using DESPOT1/DESPOT2 and the proposed dictionary approach. Banding artifact, blurring, and loss of signal in T2 maps are clearly decreased using dictionary in comparison to DESPOT2. Also, the mean was reduced considerably using the dictionary, in better agreement with the T2 reported in previous work. As with the phantoms, the T1 maps are similar for both methods.Discussion
The results show that reconstructing with the dictionary improves the quality of T2 maps in comparison with DESPOT2. This is mainly because bSSFP model used in the dictionary considers the off-resonance and banding effects in the signal model.Conclusion
A novel approach to reconstructing T2 maps using a dictionary in bSSFP acquisitions has been applied to correct off-resonance distortions. The dictionary approach improved the T2 map reconstructions in comparison to conventional DESPOT2, especially for prostate tissues. Future work will be accelerating the acquisition using SENSE and compressed sensing in combination with the better TRs, FAs, and RF phases.Acknowledgements
This work was funded by Fondecyt 1210648 and 1210747, and Millennium Science Initiative Program ICN2021_004 (iHealth)References
1. Deoni SCL, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reason Med 2003;49:515–526.
2. Deoni SCL. Transverse relaxation time (T2) mapping in the brain with off-resonance correction using phase-cycled steady-state free precession imaging. J Magn Reson Imaging 2009;30:411–417.
3. Klingebiel, Maximilian, et al. "Value of T2 Mapping MRI for Prostate Cancer Detection and Classification." Journal of Magnetic Resonance Imaging (2022).
4. Rangwala, Novena A., et al. "Optimization and evaluation of reference region variable flip angle (RR‐VFA) and T1 Mapping in the Prostate at 3T." Journal of Magnetic Resonance Imaging 45.3 (2017): 751-760.
5. G. Captur et al., “A T1 and ECV phantom for global T1 mapping quality 708assurance: The T1 mapping and ECV standardisation in CMR (T1MES) 709program,” J. Cardiovascular Magn. Reson., vol. 18, no. S1, p. W14, 710 Jan. 2016.
Figures