1597
High-performance, Shielding-free MSK Imaging at 100mT Enabled by Retrospective Active EMI Cancellation1Center for Adaptable MRI Technology (AMT Center), Department of Biomedical Engineering, University of Basel, Allschwil, Switzerland, 2AMT Center, Institute of Medical Sciences, School of Medicine, Medical Sciences & Nutrition, University of Aberdeen, Aberdeen, United Kingdom
Synopsis
Keywords: Low-Field MRI, Low-Field MRI
Electromagnetic interference cancellation method EDITER is implemented in an unshielded low-field MRI scanner at 100mT by addition of only two EMI detection coils to the acquisition chain. The method’s effectiveness is illustrated in high resolution MSK images of healthy volunteer’s hands. Full 3D images with sub-millimeter in-plane or isotropic millimeter voxel dimensions can be obtained in clinically acceptable acquisition times. This approach, in combination with acceleration strategies, opens perspectives in MSK imaging in the field with portable scanners and/or in low resource environments.
Introduction
Low magnetic field strengths offer opportunities to scale down the physical footprints of MRI scanners in search of enhanced accessibility. Electromagnetic shielding typically used in clinical units allows for considerable gain in detection sensitivity that translates in added complexity and altered flexibility. Alternatives are proposed to evade this need in recent low-field MRI initiatives, including solutions based on compact Faraday cage-like shielding1, conductive cloth material2, to most recent active noise cancelling strategies3,4. Here, we present musculoskeletal (MSK) imaging in healthy volunteers at 100mT in our resistive, biplanar MRI system solely relying on active EMI cancellation based on External Dynamic InTerference Estimation and Removal (EDITER)4. We assess the value of active noise cancellation in a potentially limited resource environment where access to a multitude of EMI detection coils (EDCs) might not be feasible. With only two or three receive channels, i.e., one or two used for EMI cancellation and one (Tx/Rx) for the primary rf-coil, we could achieve isotropic millimeter resolution and in-plane sub-millimeter resolution in 3D in the hand/wrist, without undersampling.Methods
An inductively matched (50ohm) transceiver coil was used to image the hand and wrist at 4.25MHz, with S11 attenuation: -31dB and bandwidth (BW): 18.8kHz. EDITER was implemented with a maximum of two EDCs, tuned to the same frequency as the primary transceiver rf-coil. Bandwidth of the EDCs was ~60kHz to capture sufficient readout bandwidth in diverse imaging scenarios. EDCs were respectively oriented parallel (EDC 1) and perpendicular (EDC 2) to the primary rf-coil’s B1-field. The optimized location of the EDCs was ~2.5m away from the system’s iso-center, sitting on our magnet power supply (Figure 1). A fully sampled 3D gradient echo (GRE) sequence was used with parameters as follows: TE/TR = 20/80ms, flip angle (FA) = 80°, BW = 20kHz, matrix size 512x147x19, voxel resolution 0.8x0.8x3.0mm3, number of averages (NA) = 3, for a total acquisition time of 11min10s. Fully sampled 3D balanced steady state free precession (bSSFP) was also used with parameters: TE/TR = 7.3/14.1ms, FA = 70°, BW = 20kHz, matrix size 128x117x57, isotropic voxel resolution 1.0x1.0x1.0mm3, NA = 10, for a total acquisition time of 15min37s.Results
Figure 2 shows one slice from a full 3D GRE dataset in a healthy volunteer’s hand, before and after EDITER processing using one or two EDCs. Maximum signal-to-noise ratio in the entire image was found as 11±3 in the original image acquired with only the primary rf-coil, 42±4 for EDITER with EDC 1, and 49±5 for EDITER with EDC 1 + 2. Figure 3 shows 3-axis images of a healthy volunteer’s hand with bSSFP, before and after EDITER processing with two EDCs.Discussion
Showing good EMI cancellation performance, a major advantage of EDITER lies in its simplicity of use. Overall scan times remain unchanged compared to the same acquisition without EDITER (i.e., there is no need for additional noise-only acquisition windows in the sequence), and the demand on computer hardware is reasonably low (no need for dedicated GPU) with a processing time of only a few seconds on a regular desktop computer. By carefully optimizing the position and orientation of a single EDC, most of the EMI present in the original image could be removed. We found that EDC 1, oriented parallel to the primary rf-coil’s B1-field, was most effective. Adding a second EDC improved image quality more subtly. This indicates that for low resource scenarios or highly portable scanners, active EMI cancellation could be implemented with very little added system complexity and might prove extremely useful to improve image quality.Although results are already striking, EMI induced artifacts may not always be fully removed, and noise picked up by the EDCs only (and not by the primary rf-coil), may appear in the reconstructed images (Figure 2). Adjustments in placement and orientation of the second EDC might help to further improve performance. Further, deep learning approaches to map noise in the primary coil may be used3, but at the cost of added computing time and complexity. Additionally, EMI introduced through the subject’s body is not yet considered, and might be compensated for using suitable detectors such as electrodes placed on the subject4.
Conclusion
The presented images show drastically reduced noise thanks to application of EDITER, enabling clinically relevant imaging in the hand and wrist with fully sampled Cartesian acquisition schemes. At 100mT, we could obtain sub-millimeter in-plane voxel size with 3D GRE sequences, and isotropic millimeter resolution with 3D bSSFP sequences, in ~11min and ~16min, respectively. We show that a single EDC greatly improves image quality, further stressing its value for low resource application scenarios or enhanced portability for imaging in the field. Further acceleration of the total acquisition time can easily be envisioned through non-Cartesian acquisition or modern undersampling strategies, showing great promise for future compact, shielding free low-field MRI systems.Acknowledgements
Swiss National Science Foundation Grant No. 186861.
Swiss National Science Foundation Grant No. 198905.
References
(1) He, Y.; He, W.; Tan, L.; Chen, F.; Meng, F.; Feng, H.; Xu, Z. Use of 2.1 MHz MRI Scanner for Brain Imaging and Its Preliminary Results in Stroke. J. Magn. Reson. 2020, 319, 106829. doi: 10.1016/j.jmr.2020.106829.
(2) O’Reilly, T.; Teeuwisse, W. M.; Gans, D.; Koolstra, K.; Webb, A. G. In Vivo 3D Brain and Extremity MRI at 50 MT Using a Permanent Magnet Halbach Array. Magn. Reson. Med. 2021, 85 (1), 495–505. doi: 10.1002/mrm.28396.
(3) Liu, Y.; Leong, A. T. L.; Zhao, Y.; Xiao, L.; Mak, H. K. F.; Tsang, A. C. O.; Lau, G. K. K.; Leung, G. K. K.; Wu, E. X. A Low-Cost and Shielding-Free Ultra-Low-Field Brain MRI Scanner. Nat. Commun. 2021, 12 (1), 7238. doi: 10.1038/s41467-021-27317-1.
(4) Srinivas, S. A.; Cauley, S. F.; Stockmann, J. P.; Sappo, C. R.; Vaughn, C. E.; Wald, L. L.; Grissom, W. A.; Cooley, C. Z. External Dynamic InTerference Estimation and Removal (EDITER) for Low Field MRI. Magn. Reson. Med. 2022, 87 (2), 614–628. doi: 10.1002/mrm.28992.
Figures
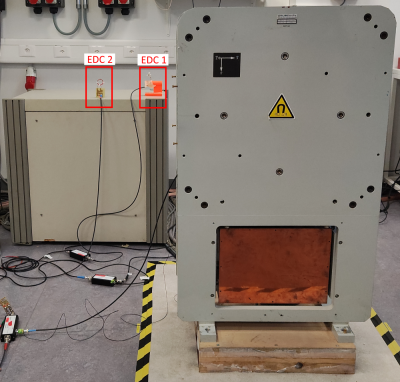
Figure 1: The measurement setup used for this work. The two EDCs are located on the magnet power supply ~2.5m away from the MRI system’s iso-center. EDC 1 and EDC 2 are oriented parallel and perpendicular to the primary rf-coil’s B1-field, respectively.
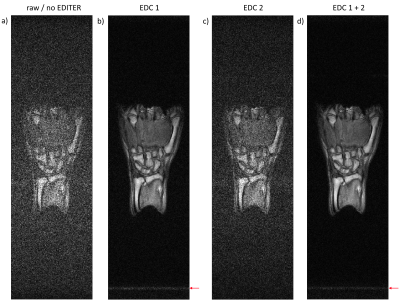
Figure 2: One slice of a 3D GRE image of a healthy volunteer’s hand. a) Original image acquired with the primary rf-coil. b) & c) Images after EDITER processing with only EDC 1 and EDC 2, respectively. d) Image after EDITER processing with both EDCs combined. Red arrows highlight some EMI artifacts present after EDITER processing.
Sequence parameters: Matrix dimension: 512x147x19. Voxel size: 0.8x0.8x3.0mm3. TE/TR: 20/80ms. BW: 20kHz. FA: 80°. NA: 3. Total acquisition time: 11min10s.
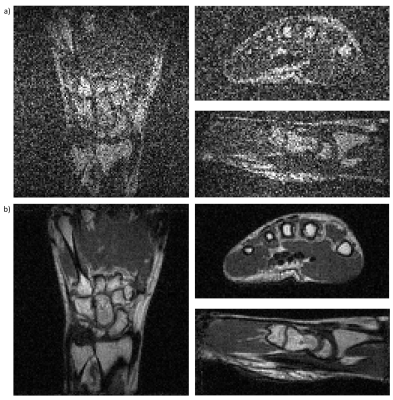
Figure 3: Selected slices along the three main axes of a 3D bSSFP image of a healthy volunteer’s hand with 1.0mm3 isotropic resolution acquired at 100mT. a) Original image as acquired only with the primary transceiver rf-coil. b) Resulting image after retrospective active EMI cancellation using EDITER with two EDCs.
Sequence parameters: Matrix dimension: 128x117x57. Voxel size: 1.0x1.0x1.0mm3. TE/TR: 7.3/14.1ms. BW: 20kHz. FA: 70°. NA: 10. Total acquisition time: 15min37s.