1590
Overhauser-enhanced MRI at ultra-low fields: a new system for imaging of free radicals in rodents
Dahmane Boudries1, Philippe Massot1, Elodie Parzy1, Seda Seren1, Philippe Mellet2, Jean-Michel Franconi1, Sylvain Marque3, Florian Fidler4, Stefan Wintzheimer5, Markus Mützel5, and Eric Thiaudiere1
1CNRS, Bordeaux, France, 2INSERM, Bordeaux, France, 3CNRS, Marseille, France, 4Fraunhofer, Würzburg, Germany, 5Pure devices, Rimpar, Germany
1CNRS, Bordeaux, France, 2INSERM, Bordeaux, France, 3CNRS, Marseille, France, 4Fraunhofer, Würzburg, Germany, 5Pure devices, Rimpar, Germany
Synopsis
Keywords: Low-Field MRI, Molecular Imaging, Ultra low field, OMRI, DNP, Magnetic prepolarization
A new MRI system at Ultra-low field (206 µT) was designed for Overhauser-enhanced MRI. It allows both conventional MRI with pre-polarization at 20 mT and Dynamic Nuclear Polarization in the 70MHz range in living rats. Anatomical images in 3D allowed a correct visualization of the rat body shape. OMRI with injected or instilled stable and non-toxic nitroxides permitted to detect the free radicals in the lungs, the kidneys and the bladder. The work opens the way of molecular imaging of abnormal proteolysis in the context of a variety of diseases in large animals.Purpose / Introduction
Molecular imaging will allow the development of personalized medicine. Innocuousness of MRI and its natural contrasts makes it a good candidate, provided sensitivity and specificity issues are addressed. For instance abnormal proteolysis could be a target for molecular imaging in a variety of diseases. Recent developments of OMRI at low-field (0.19T) with a dedicated nitroxide allowed the detection of neutrophil elastase activity associated with inflammation in the lungs of mice.1 In order to transfer this technique to large samples and furthermore to humans, it is necessary to reduce the EPR irradiation frequency, namely 5.4GHz at 0.19T. One solution consists in reducing the magnetic field. For this purpose, an original instrument with a new system was implemented within the European project PrimoGAIA. A first version of this equipment is intended to rat-sized imaging at ULF (ultra-low field, 206µT) with 70MHz EPR frequency for the nitroxides used. The purpose of this study was to evaluate the feasibility of ultra-low-field OMRI to observe the biodistribution of free radicals in living rats.Subject & Method
The instrumental PrimoGAIA (First-Kernel) ULF system (Fig.01) integrates: a B0 cage able to create a static magnetic field in any direction from 0 to 206μT and compensate at the same time the Earth’s magnetic field; a 3D gradients setup for space encoding, with maximum strengths of: GX = 488µT/m, GY = 647µT/m and GZ = 238µT/m; a magnetic pre-polarization unit (switchable solenoid coil producing 20mT) ; an 1H NMR unit (transmit/receive channel at 8.79kHz ; 110mm length and 80mm diameter low-frequency transmit-receive ”gradiometer” coil) ; an EPR unit (RF transmitter, Birdcage coil (8legs, D = 62mm, L = 95mm) designed with Comsol® Multiphysics to operate at 72MHz at 206uT ; a hardware controller run with Matlab® software.Sequences
All NMR experiments were carried out at 206µT and included a preparation step, either pre-polarization at 20mT for anatomical imaging, or EPR irradiation for OMRI. For in-vitro imaging on a multi-compartment phantom with various TOPCA concentrations (0.1 to 1mM), a classical Spin Echo (TR/TE = 1730/85ms) sequence was used with 1.5s magnetic pre-polarization and bSSFP (TR/TE = 115/57.5ms ) sequence for OMRI with EPR applied throughout the experiment. In vivo imaging was carried out in anesthetized (ketamine/xylazine) Wistar female rats (body weight 250g). Rats were either i.v. injected with 1ml TOPCA at 200mM or instilled intratracheally with 100µl 3-carboxy-proxyl at 100mM. Because of short apparent T2 (~100ms) as compared with the achievable echo times, a ZTE (TR/TE = 245/13ms) sequence was preferred. This sequence was used both for anatomical images using magnetic 0.5s pre-polarization and for OMRI in rat kidneys, bladder and lungs.
Results
In-vitro imagingThe 3D image (Fig.02) using magnetic pre-polarization at 4mm resolution showed a clear delineation of the 5mm vials in the multi-compartment phantom. The signal dependence as a function of nitroxide concentration was due to incomplete magnetization with 1.5s pre-polarization times for longer T1. SNR varied from 8.5 to 15. The 3D-OMRI acquisition with bSSFP using continuous EPR allowed detection of nitroxides at concentrations as low as 0.1mM. The SNR ranged from 7.5 to 19.
In-vivo imaging
With magnetic pre-polarization the shape of the full body of the rat could be acquired in 3D from 3 separate experiments (Fig.03). The average SNR was 9.6. OMRI performed either in the abdomen or in the chest allowed a clear visualization of nitroxides in the kidneys (Fig.04.C-D), the bladder (Fig.04.B-D) and in lungs(Fig.04.A). The SNR were in the range 3-5.
Discussion / Conclusion
The system is cost-effective and allows both conventional 3D-imaging with pre-polarization and 3D-OMRI in living rodents. The nitroxides used were stable and not toxic. No tissue heating issue was encountered, thanks to the low EPR frequency. This study opens the way of future molecular molecular imaging of proteolysis in the context of inflammatory diseases, e.g. in lungs or kidneys. Further developments are in progress to translate this approach on larger animals.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 863099.References
- Rivot, Angélique, et al. "Magnetic Resonance Imaging of Protease-Mediated Lung Tissue Inflammation and Injury." ACS omega 6.23 (2021): 15012-15016.
Figures
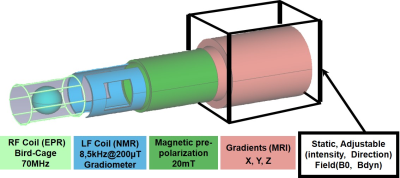
Figure 01: Schematic of the scanner assembly
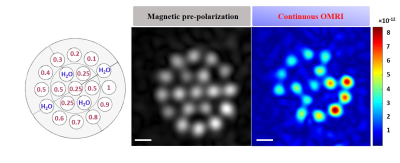
Figure 02: In vitro 3D image of
the phantom on the left, 1mL tubes with different concentrations of contrast agent
(from 0.1 to 1mM), with 1mL water tubes in between. On the right is a 3D image
obtained using magnetic prepolarization and OMRI with the same spatial
acquisition parameters: FOV of 72×72×56mm3 and resolution of 4×4×6mm3.
For magnetic prepolarization timing, the TE/TR were 85/1730ms, including
prepolarization, and the total acquisition time was 43min with 6 averages. In
OMRI, the TE/TR were 57.5/115ms and the acquisition time was 37s with 4
averages.
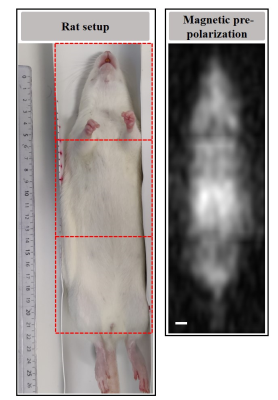
Figure 03: In vivo 3D image of the rat using magnetic prepolarization, showing a single coronal slice. The image displayed is the result of the three images acquired in an equivalent way (3 zones: Head-Shoulders, Thorax-abdomen and
Pelvis-tail side), with acquisition parameters: resolution 3×3×6mm3, FOV 81×81×60mm3, TE/TR 13/745ms, including a polarization pulse with a readout of 184ms, for a total acquisition time of 30min and 90min for the whole rat.

Figure 04: OMRI 3D images of the
kidney (C, D) acquired with the same acquisition parameters as in magnetic
prepolarization, with a total
acquisition time of 560s, in image (C), the t0 which is the beginning of
acquisition is 0s, and for (D) was 15min after injection. For lung imaging,
acquisition was performed at a resolution of 5.7×5.7×6mm3 with the
same previous FOV, the TE/TR is 13/208ms, and the total acquisition time is
168s. In image (A), the t0 is 2min, and in image B, the t0 is 15min the
FOV centered on the bladder with the same acquisition parameters as the kidney.
DOI: https://doi.org/10.58530/2023/1590