1584
Automatic bundle-specific white matter fiber tracking tool for language related glioma resection1Department of neurosurgery, Huashan Hospital Fudan University, Shanghai, China, 2Institute of Neuroscience, National Yang Ming Chiao Tung University, Hsinchu, Taiwan, Hsinchu, Taiwan, 3Department of neurosurgery, Taipei Veterans General Hospital, Taipei, Taiwan, 4MR Collaboration, Siemens Healthineers Ltd, Shanghai, China, 5MR Collaboration, Siemens Healthineers Ltd, shanghai, China, 6Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, shanghai, China
Synopsis
Keywords: Tumors, Diffusion Tensor Imaging
Novel fiber tracking technology, based on diffusion imaging, can objectively reveal and visualize three-dimensional white matter tracts; through the cooperation of intraoperative navigation, it can help achieve maximum resection under the premise of ensuring function. We used an in-house developed software (DiffusionGo) specially designed for neurosurgeons. The fiber tracking result using DiffusionGo showed robust consistency with the surgical findings. We believe that this fully automatic processing pipeline provides the neurosurgeon with a solution that may reduce time costs and operating errors and improve care and surgical procedure quality across different neurosurgical centers.Introduction
Glioma is the most common malignant intradural tumor. Surgery is the first-line treatment for debulking tumors and obtaining tissues for pathology analysis. However, glioma grows infiltratively along fiber tracts, making it difficult to determine the tumor boundary. Extended resection may impair eloquent brain areas and cause functional disorders such as hemiplegia and aphasia. Therefore, precise tracing of tumor boundary is the key to balancing the survival and quality of life of glioma patients. Diffusion tensor imaging (DTI) is a noninvasive technique that can probe the molecular diffusivity of water within the white matter to reflect the intravoxel architecture by measuring the water self-diffusion tensor, which has been used to reveal and visualize three-dimensional white matter tracts and provides crucial information to neurosurgeons for neurosurgical planning and navigation[1]. We used an in-house developed software (DiffusionGo) [2] specially designed for neurosurgeons. The fiber tracking result using DiffusionGo showed robust consistency with the surgical findings.Material and methods
We report two patients with preoperatively imaging-diagnosed gliomas who underwent surgical resection and had MRI scan at Huashan Hospital (Shanghai, China) and at First Affiliated Hospital of Fujian Medical University (Fuzhou, Fujian, China), respectively. For the first case, images were acquired on a 3T MRI scanner (Magnetom Verio; Siemens, Erlangen, Germany) with a 12-channel head coil at Huashan Hospital, including three-dimensional T1-weighted images and diffusion-weighted images (DWI). For the second case, T1w images and DWI were acquired on a 3T MRI scanner (Siemens Magnetom Prisma, Erlangen, Germany) at the First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China, using a 64-channel head array coil. Imaging protocols was listed in Figure 1. DiffusionGo integrates a preprocessing pipeline with a fully automatic multiple assigned criteria algorithm for bundle-specific tractography using DTI data based on anatomical connectivity [2]. First, all images were coregistered with DWI by using Advanced Normalization Tools (ANTs, http://stnava.github.io/ANTs/). All DWIs underwent diffusion preprocessing pipeline and DTI model fitting with MRtrix3 (https://www.mrtrix.org) and FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). A patent-protected multiple assigned criteria (MAC) algorithm [2] for fiber tracking was used. The motor pathway (corticospinal tract, CST), language pathway (arcuate fasciculus, AF, superior longitudinal fasciculus, SLF, frontal aslant tract, FAT, inferior longitudinal fasciculus, ILF, inferior fronto-occipital fasciculus, IFOF, and uncinate fasciculus, UF), and visual pathway (optic radiation, OR) were segmented automatically. The potential false-positive tracts were manually removed. The workflow is provided in Figure 2. Multimodality-guided awake surgery under electrophysiology monitoring for language function mapping and preservation was used. Speech arrest was defined as discontinuing number counting without simultaneous motor response by direct cortical stimulation (DCS).Results and discussion
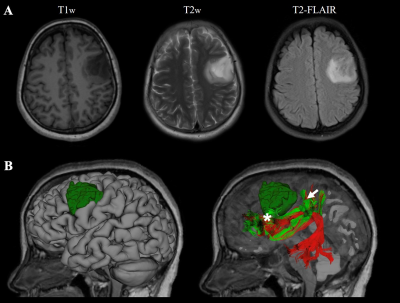
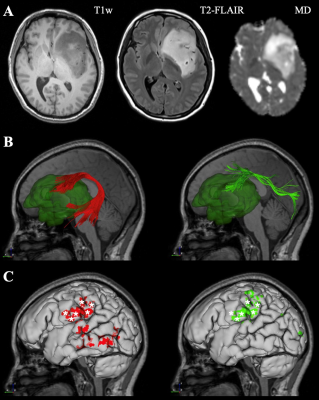
The first case was a 42-year-old woman with left frontal-temporal-insular lobe astrocytoma in Huashan Hospital. The lesion is close to the speech output language area with high surgical risk (Figure 3A). Considering the tumor might not be malignant and is sensitive to subsequent radiotherapy and chemotherapy, we focused on functional protection to maintain a relatively high living quality for the patient. Figure 2B shows the reconstructed AF and SLF-II, the major white matter tracts adjacent to the lesion. In the language mapping phase, we found that the eloquent area of speech arrest was located in the classical Broca's area as the terminal territory of our reconstructed AF (Figure 3C). The surgery was conducted under awake surgery, and there was no language dysfunction during the procedure. The second case was conducted at the First Affiliated Hospital of Fujian Medical University. This 41-year-old female suffered from recurrent seizures attack for 4 years. Conventional MR indicates a left frontal-insular lesion of 3.8 cm, which is the high signal in T2WI and not enhanced after contrast (Figure 4A). Preoperative DTI showed the AF and SLF were located below and behind the tumor, respectively (Figure 4B). In the language mapping phase using DCS, we found that the eloquent area of speech arrest was located in the terminal territory of our reconstructed AF and SLF (Figure 4B). The surgery was conducted under awake surgery. Since there was no clear boundary between the tumor and the eloquent area and SLF behind the tumor, a subtotal resection was performed. There was no language dysfunction during the whole procedure.Conclusion
We demonstrated the application of DiffusionGo in two language-related language related glioma resection. The fully automatic processing pipeline may provide the surgeon a solution to reduce operating error. We believe that this promising technique can improve care quality and surgical procedure quality across different facilities.Acknowledgements
No acknowledgement found.References
[1] Basser PJ: Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine 1995, 8(7):333-344.
[2] Lin C-P, Chong ST, Lo C-Y, Huang C-C: Method and apparatus of fiber tracking, and non-transitory computer-readable medium thereof. In.: Google Patents; 2019.
Figures

Demographic of a patient with left frontal-temporal lobe astrocytoma.
Case 1, a 42-year-old woman with left frontal-temporal lobe astrocytoma (WHO grade II) in MR images (A). The relationship between language-related fiber tracts (AF in red and SLF-II in greed color) and tumor (dark green color) were shown in (B). The cortical termination of each tract was projected on the cortical surface (C). The eloquent area of intro-operative speech arrest (DCS) was marked with stars.