1583
Determination of Meningiomas Consistency by Histogram Analysis of Different Models of Diffusion-Weighted MR Imaging
Lingmin Zheng1, Zongmeng Wang1, Danjie Lin1, Yang Song2, Lin Lin1, and Yunjing Xue1
1Fujian Medical University Union Hospital, Fuzhou, China, 2MR Scientific Marketing, Siemens, Healthineers Ltd, Shanghai, China
1Fujian Medical University Union Hospital, Fuzhou, China, 2MR Scientific Marketing, Siemens, Healthineers Ltd, Shanghai, China
Synopsis
Keywords: Tumors, Diffusion/other diffusion imaging techniques
The consistency of Intracranial tumors is crucial to determine the required surgical instruments as well as affecting the outcome of surgery, but no specific feature of conventional MRI is reliable in predicting the consistency of tumors. Histogram analysis of diffusion parameters has been successfully used in predicting the grade, subtype, and proliferative activity of meningiomas. This study prospectively evaluated and compared the potential of various diffusion metrics obtained from the mono-exponential model (MEM), bi-exponential model (BEM), and stretched exponential model (SEM)-based diffusion-weighted imaging (DWI) in predicting consistency of meningiomas. It was found that different models of DWI (MEM, BEM, and SEM) are useful in the differentiation between soft and hard meningiomas. However, Alpha obtained from SEM and fast-ADC from BEM are more promising diffusion parameters for predicting the consistency of meningiomas.Purpose
Meningiomas are one of the most common primary brain tumors.1 Although conventional MRI provides several identifiable features for meningiomas, no specific feature is reliable in predicting the consistency of the tumor.2 Histogram analysis of diffusion parameters have been successfully used in predicting the grade, subtype, and proliferative activity of meningiomas.3 This study prospectively evaluated and compared the potential of various diffusion metrics obtained from the mono-exponential model (MEM), bi-exponential model (BEM), and stretched exponential model (SEM)-based diffusion-weighted imaging (DWI) in predicting consistency of meningiomas.Methods
Forty-seven consecutive patients with histopathologically confirmed meningiomas were prospectively enrolled in this study. MRI scans were performed on a 3.0T MR scanner with an eight-channel receiver head coil. DWI used a SE-EPI diffusion sequence in the axial plane (TR/TE = 5,000/84.6 ms, slice thickness/ slice gap = 5 mm/0 mm, FOV = 24 cm, matrix = 192 × 192, number of sections = 30, FOV = 24 cm. Twelve b values from 0 to 3000 sec/mm2 (0, 50, 100, 150, 200, 300, 500, 800, 1000, 1500, 2000, and 3000 sec/mm2). The DWI data were obtained and transferred to a workstation (Advantage Workstation 4.6) for processing. Parameter maps were generated automatically by the MADC program in the Functool software. Two neurosurgeons evaluated the tumor consistency and classified them as soft and hard groups. A Volume of interest was placed on the preoperative MR diffusion images to outline the whole tumor area by using ITK-SNAP (Version 3.6.0). Histogram parameters (Mean, Median, 10 Percentile, 90 Percentile, Kurtosis, Skewness) were extracted by Feature Explorer (Version 0.5.3) from 6 diffusion maps including Apparent diffusion coefficient (ADC), pure molecular diffusion (slow-ADC), pseudo-diffusion coefficient (fast-ADC), fraction of fast ADC (f), water molecular diffusion heterogeneity index (Alpha), and distributed diffusion coefficient (DDC), respectively. Comparisons of the two groups were made by Student's t-Test or Mann-Whitney U test. Parameters with significant differences between the two groups were included for Receiver operating characteristic analysis. DeLong test was used to compare AUCs.Results
Seventeen of 47 tumors were included in the hard group by neurosurgeons during the operation, and the rest were classified as soft tumors. DDC, fast-ADC, and ADC 10 Percentile were significantly lower in hard tumors than in soft tumors (P ≤ 0.05). Alpha and fraction of fast-ADC 90 percentile were significantly higher in hard tumors than in soft tumors (P < 0.02). As for other parameters, no significant difference was found. Representative cases of soft and hard meningioma are shown in Figs. 1. Fig. 2 shows that the Alpha 90 percentile yielded the highest AUC of 0.876. Fast-ADC 10 percentile had a relatively lower AUC value, followed by a fraction of fast-ADC 90 Percentile, DDC 10 Percentile and ADC 10 Percentile. Alpha 90 percentile and fast-ADC 10 Percentile had greater AUC values than a fraction of fast-ADC 90 Percentile, DDC, and ADC 10 Percentile (P ≤ 0.05).Discussion and conclusion
Our results demonstrated that the differentiation of soft and hard meningiomas is feasible by different models of DWI. Generally, we found that the histogram parameters (10, 90 Percentile) performed better than the conventional parameters like Mean or Median. DDC and ADC 10 Percentile were significantly lower in hard tumors than in soft tumors. It is probably due to the higher cell density of hard tumors. Fast-ADC 10 Percentile was significantly lower in hard tumors than that in soft tumors, while fraction of fast-ADC 90 percentile was significantly higher in hard tumors than that in soft tumors. It suggests the potential correlation between tumor microperfusion and consistency. Alpha 90 percentile was significantly higher in hard tumors than in soft tumors. This may intimate that hard tumors are more homogenous than soft tumors at the intravoxel level. In addition, Alpha and fast-ADC have significantly better diagnostic performances than the diffusion-related metric. It may indicate that intratumoral heterogeneity and perfusion contribute more to tumor consistency than cell density.Acknowledgements
No acknowledgement found.References
1. Kurokawa R, Kurokawa M, Baba A, et al. Major Changes in 2021 World Health Organization Classification of Central Nervous System Tumors. Radiographics. 2022;42(5):1474-1493.
2. Yao A, Pain M, Balchandani P, Shrivastava RK. Can MRI predict meningioma consistency?: a correlation with tumor pathology and systematic review. Neurosurg Rev. 2018;41(3):745-753.
3. Cao T, Jiang R, Zheng L, et al. T1 and ADC histogram parameters may be an in vivo biomarker for predicting the grade, subtype, and proliferative activity of meningioma. Eur Radiol. 2022;10.1007/s00330-022-09026-5.
Figures
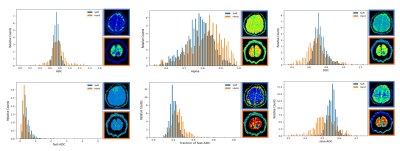
Fig. 1: Histograms of ADC, Alpha, DDC, fast-ADC, fraction of fast-ADC and slow-ADC from a soft meningioma in a 48-year-old woman (blue) and a hard meningioma in a 54-year-old woman (orange). The corresponding diffusion maps are on the right side of each histogram (the soft tumor with a blue border, the hard tumor with a orange border).
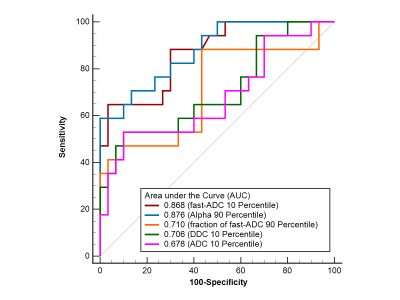
Fig.2: ROC curves of histogram parameters to differentiate soft and hard meningiomas.
DOI: https://doi.org/10.58530/2023/1583