1580
Arterial spin labeling MRI can predict prognosis of patients with newly-diagnosed primary CNS lymphoma1Department of Radiology, Huashan Hospital, Fudan University, Shanghai, China, 2Department of Hematology, Huashan Hospital, Fudan University, Shanghai, China, 3Department of Radiology, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China, 4Eye Institute, Eye and ENT Hospital, College of Medicine, Fudan University, Shanghai, China, 5Department of Neurosurgery, Huashan Hospital, Fudan University, Shanghai, China, 6GE Healthcare, Shanghai, China
Synopsis
Keywords: Tumors, Arterial spin labelling, primary CNS lymphoma, cerebral blood flow, prognostic factor
PCNSL is a heterogeneous and aggressive non-Hodgkin lymphoma with poor prognosis. The present study revealed that 3D-ASL MR perfusion imaging-derived rCBF values pre- and posttreatment can be used as predictors for patients with newly diagnosed PCNSL. Furthermore, this is the first study identifying rCBFmean as an independent predictor for overall survival. Different from the traditional prognostic scoring system, the study provided a noninvasive MRI technique that requires no contrast agent or radiation exposure. Inclusion of the 3D-ASL sequence in routine MRI protocols is highly recommended to help to optimize individualized treatment and improve prognosis.Introduction
Primary central nervous system lymphoma (PCNSL) is a highly heterogeneous and aggressive non-Hodgkin’s lymphoma with poor prognosis1-5, and its prognostic scoring system based on clinical parameters is controversial6,7. Three-dimensional arterial spin labeling (3D-ASL) MRI has the advantages of no intravascular contrast agent, non-invasiveness, and no radiation exposure. Several studies8-11 have demonstrated the value of relative cerebral blood flow (rCBF)-derived biomarkers, obtained using 3D-ASL as predictors of grade and prognosis in gliomas.The present study aimed to evaluate the prognostic value of 3D-ASL-derived relative cerebral blood flow (rCBF) as biomarkers and clinical factors in patients with newly-diagnosed PCNSL.
Methods
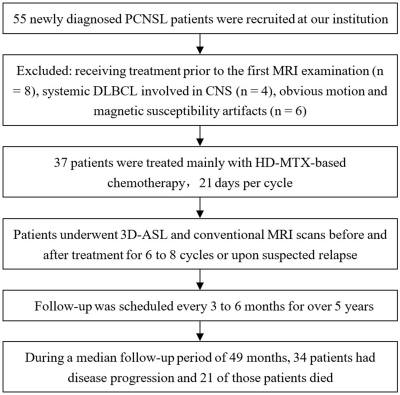
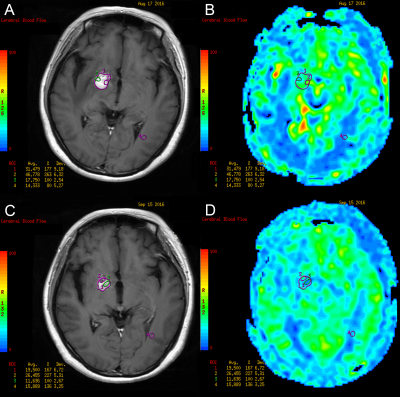
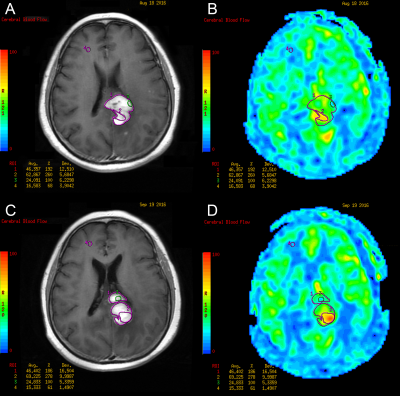
This retrospective study enrolled patients with newly-diagnosed PCNSL at Huashan Hospital, Fudan University between May 2015 and May 2017. All cases underwent 3D-ASL and conventional MRI examination on a 3T system (Signa HDxt, GE Medical Systems, Milwaukee, WI, USA) prior to each chemotherapy for 4-8 cycles until the enhanced lesions disappeared. Thereafter, all cases underwent routine sequential follow-up of MRI scan every 3-6 months. When there were signs of relapse and progression, 3D-ASL sequence was added.The 3DASL module in Functool 9.4.05 software provided by GE advantage workstation (AW) 4.6 workstation was used for image post-processing. The greyscale image of contrast-enhanced T1WI and pseudo-color image of 3D-ASL-CBF were used to perform anatomic and functional image fusion, and regions of interest (ROIs) of the tumor were outlined manually. The ROI of 40-60mm2 was placed on the normal white matter of the contralateral frontal/parietal lobe. The cerebral blood flow (CBF) of the tumor divided by the CBF of the normal white matter area was used as the rCBF for standardization. The mean, maximum, and minimum values of rCBF were outlined and calculated.
All patients were followed-up by telephone every 3-6 months for more than five years (from August, 2015 to December, 2020). 3D-ASL parameters and clinical characteristics about progression-free survival (PFS) and overall survival (OS) were calculated by log-rank test of Kaplan–Meier and Cox regression analysis of Stata statistical software.
Results
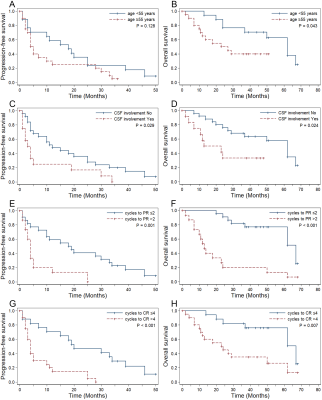
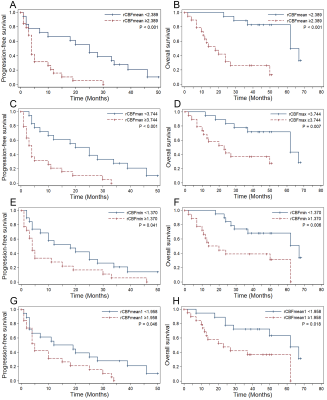
Thirty-seven patients (mean age, 53 years ± 11 [standard deviation]; range, 20–76 years; 29 men) were included. All patients were pathologically proven diffuse large B-cell lymphoma (DLBCL) and treated with high-dose methotrexate (HD-MTX)-based immuno-chemotherapy. During a median follow-up period of 49 months (range, 2–68 months), 34 of 37 patients (91.89%) suffered disease progression (PD). In total, 21 of the 37 patients (56.76%) died. The median PFS and OS were 10 months and 50 months. The 2-, 3- and 4-year PFS rates were 24.32%, 13.51%, and 5.07%. The 2-, 3- and 5-year OS rates were 62.16%, 56.76%, and 29.41%.The sites most involved by tumors were the deep nuclei, corpus callosum, and periventricular white matter; the frontal lobe was the next most common site involved. Before treatment, lesions were solitary in 14 patients, multifocal in 21 patients, and diffuse infiltration of the cranial meninges in 2 patients. Cytologic examination of cerebrospinal fluid (CSF) was performed in 29 cases at the initial stage, identifying malignant cells in 12 patients, and in 32 cases throughout the illness, identifying malignant cells in 16 patients. Thirty-three of 37 patients (89.19%) were responders, including complete remission (CR) in 31 cases and partial remission (PR) in 2 cases. In univariate analysis, inferior PFS and OS were associated with CSF involvement, failure to achieve PR and CR in two and four cycles, rCBFmean ≥2.389, rCBFmax ≥3.744, rCBFmin ≥1.370, and rCBFmean1 ≥1.958. Patients with rCBFmean1 higher than rCBFmean had poorer PFS. Predictors of shorter OS were age ≥55 years, Karnofsky Performance Score (KPS) <50, and rCBFmeanPR ≥1.981. Multivariate analysis suggested that KPS <50 (HR, 0.21; P = .004), CSF involvement (HR, 4.18; P = .022), failure to achieve CR in four cycles (HR, 3.54; P = .020), and higher rCBFmean (HR, 4.20; P = .033) were independent factors for adverse OS.
Discussion
To our knowledge, this is the first study to demonstrate significantly divergent outcomes among patients with PCNSL by 3D-ASL-derived median rCBF-defined risk stratifications of hyperperfusion and hypoperfusion groups. The heterogeneous microvessel density and the degree of neovascularization provide a theoretical basis for using CBF to classify PCNSL. ASL-derived CBF values have been utilized successfully to predict clinical outcomes in gliomas and to differentiate PCNSL from gliomas and brain metastases9, 11-14.CSF involvement may reflect tumor burden, leptomeningeal involvement, or even disease severity. Our results, showing CSF involvement as an adverse predictor for PFS and OS, are consistent with some15, 16, but not all17-19, previous studies. Our results demonstrated failure to achieve CR in four cycles was an independent risk factor for both PFS and OS, which are similar to the results of a previous study by Pels et al20 but different from those of the study by Tabouret et al21.
Conclusion
This study demonstrated that 3D-ASL-derived biomarkers of rCBFmean, rCBFmax, and rCBFmin can predict the prognosis of patients with PCNSL. These biomarkers can guide the selection of individual therapeutic regimens for patients, help clinicians with appropriate surveillance and management based on risk group, and improve prognosis. The inclusion of 3D-ASL sequence in routine MRI protocols for patients with PCNSL is highly recommended.Acknowledgements
We greatly acknowledge Wenting Rui, Liangsong Shen, and Yingfeng Zhu from Huashan Hospital for data collecting. We are grateful to Xiaoqin Yang and nurses from Huashan Hospital for their help indwelling intravenous needles. We express our gratitude to hematologists of Department of Hematology, Huashan Hospital, Fudan University for their contributions to this work.References
1. Yang H, Xun Y, Yang A, et al. Advances and challenges in the treatment of primary central nervous system lymphoma. J Cell Physiol. 2020;235(12):9143-9165.
2. Grommes C, DeAngelis L. Primary CNS Lymphoma. J Clin Oncol. 2017;35(21):2410-2418.
3. Han CH, Batchelor TT. Diagnosis and management of primary central nervous system lymphoma. Cancer. 2017;123(22):4314-4324.
4. Sinicrope K, Batchelor T. Primary Central Nervous System Lymphoma. Neurol Clin. 2018;36(3):517-532.
5. Choi YS. Recent advances in the management of primary central nervous system lymphoma. Blood Res. 2020;55(S1):S58-S62.
6. Yuan XG, Huang YR, Yu T, et al. Primary central nervous system lymphoma in China: a single-center retrospective analysis of 167 cases. Ann Hematol. 2020;99(1):93-104.
7. Takashima Y, Kawaguchi A, Sato R, et al. Differential expression of individual transcript variants of PD-1 and PD-L2 genes on Th-1/Th-2 status is guaranteed for prognosis prediction in PCNSL. Sci Rep. 2019;9(1):10004.
8. Falk Delgado A, De Luca F, van Westen D, et al. Arterial spin labeling MR imaging for differentiation between high- and low-grade glioma-a meta-analysis. Neuro Oncol. 2018;20(11):1450-1461.
9. Pang H, Dang X, Ren Y, et al. 3D-ASL perfusion correlates with VEGF expression and overall survival in glioma patients: Comparison of quantitative perfusion and pathology on accurate spatial location-matched basis. J Magn Reson Imaging. 2019;50(1):209-220.
10. Qu Y, Zhou L, Jiang J, et al. Combination of three-dimensional arterial spin labeling and stretched-exponential model in grading of gliomas. Medicine (Baltimore). 2019;98(25):e16012.
11. Yoo RE, Yun TJ, Hwang I, et al. Arterial spin labeling perfusion-weighted imaging aids in prediction of molecular biomarkers and survival in glioblastomas. Eur Radiol. 2020;30(2):1202-1211.
12. Xi YB, Kang XW, Wang N, et al. Differentiation of primary central nervous system lymphoma from high-grade glioma and brain metastasis using arterial spin labeling and dynamic contrast-enhanced magnetic resonance imaging. Eur J Radiol. 2019;112:59-64.
13. Di N, Cheng W, Chen H, et al. Utility of arterial spin labelling MRI for discriminating atypical high-grade glioma from primary central nervous system lymphoma. Clin Radiol. 2019;74(2):165 e1- e9.
14. You SH, Yun TJ, Choi HJ, et al. Differentiation between primary CNS lymphoma and glioblastoma: qualitative and quantitative analysis using arterial spin labeling MR imaging. Eur Radiol. 2018;28(9):3801-3810.
15. Royer-Perron L, Hoang-Xuan K. Management of primary central nervous system lymphoma. Presse Med. 2018;47(11-12 Pt 2):e213-e44.
16. Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266-272.
17. Fossard G, Ferlay C, Nicolas-Virelizier E, et al. Utility of post-therapy brain surveillance imaging in the detection of primary central nervous system lymphoma relapse. Eur J Cancer. 2017;72:12-19.
18. Lin C-H, Yang C-F, Yang H-C, et al. Risk Prediction for Early Mortality in Patients with Newly Diagnosed Primary CNS Lymphoma. Journal of Cancer. 2019;10(17):3958-3966.
19. Deguchi S, Nakashima K, Muramatsu K, et al. Pretreatment intratumoral susceptibility signals correlate with response to high-dose methotrexate and progression-free survival in primary central nervous system lymphoma. J Clin Neurosci. 2019;69:43-50.
20. Pels H, Juergens A, Schirgens I, et al. Early complete response during chemotherapy predicts favorable outcome in patients with primary CNS lymphoma. Neuro Oncol. 2010;12(7):720-724.
21. Tabouret E, Houillier C, Martin-Duverneuil N, et al. Patterns of response and relapse in primary CNS lymphomas after first-line chemotherapy: imaging analysis of the ANOCEF-GOELAMS prospective randomized trial. Neuro Oncol. 2017;19(3):422-429.
Figures