1569
The Value of Diffusion Weighted Imaging in Predicting the Growth Pattern of Meningioma: A Retrospective Study
Hui Zheng1, Zongmeng Wang1, Lingmin Zheng1, Rufei Zhang1, Yang Song2, and Lin Lin1
1Fujian Medical University Union Hospital, Fuzhou, China, 2MR Scientific Marketing, Siemens, Healthineers Ltd., Shanghai, China
1Fujian Medical University Union Hospital, Fuzhou, China, 2MR Scientific Marketing, Siemens, Healthineers Ltd., Shanghai, China
Synopsis
Keywords: Tumors, Diffusion/other diffusion imaging techniques
The natural history of meningioma remains unclear and a simple, practical method is needed to identify fast growth tumors. This study evaluated correlations between clinical parameters, tumor growth rate (TGR), tumor volume doubling time (VDT), Ki-67 and relative apparent diffusion coefficient (rADC) derived from diffusion weighted imaging (DWI). The results showed that rADC was an independent predictive parameter of meningioma growth and baseline rADC had a good predictive ability for differentiating slow growth from fast growth meningioma. This suggests that in asymptomatic meningiomas, DWI might be a valuable predictive imaging method.Purpose
Individualized treatment strategies are needed for patients with meningiomas because the nature of meningiomas and the potential consequences of treatment vary widely among patients (1). The general trend in meningioma treatment is shifting from surgery to active surveillance, but its natural history remains unclear and a simple, practical method is needed to identify fast growth tumors and for more appropriate timing of surgical intervention (2). It has been reported that the ADC value of meningioma is related to the cell density and proliferation index of the tumor (3). But to the best of our knowledge, the predictive value of DWI for meningioma growth has not been investigated. We hypothesized that DWI could be a valuable diagnostic technique for predicting the growth of meningiomas. The purpose of this study is two-fold: 1) to determine whether ADC value can predict the growth of meningiomas (non-growth or growth), and 2) whether it has predictive value for tumor growth patterns (slow growth or fast growth).Methods
Between July 2011 and July 2019, asymptomatic adult patients diagnosed with meningioma by MRI and followed up in our hospital were consecutively included in this study. The inclusion criteria were as follows: (a) MR examination at least two-time points before surgery; (b) the first (baseline) MR examination including DWI. The exclusion criteria were the following: (a) patients who received any form of treatment targeting the meningioma; (b) insufficient image quality to meet the research requirements; (c) patients with neurofibromatosis type 2 (NF2); (d) time interval of two MRI examinations<12 months. The MR images were obtained on a 3.0T MR scanner according to our standard institutional protocols. Conventional MRI sequences included axial T1-weighted, axial T2-weighted, axial T2 fluid-attenuated inversion recovery, axial T2-weighted gradient-recalled echo, and axial T1-weighted images after contrast administration. DWI used a spin echo (SE)-echo planar imaging (EPI) diffusion sequence in the axial plane. The DWI was performed by applying sequentially in the x, y, and z directions with the following parameters: TR/TE, 3100/91 ms; FOV, 20 cm; flip angle, 90 degrees; slice thickness/spacing, 5 mm/1.5 mm; matrix 192 × 192; voxel size, 1.2 × 1.2 × 5 mm3; b = 0 and 1000 sec/mm2. ADC maps were obtained from these imaging data. Univariable and multivariable logistic regression analyses were performed to evaluate the tumor growth associated with clinical parameters (including sex, age, and follow-up time) and relative apparent diffusion coefficient (rADC). Correlations between tumor growth rate (TGR), tumor volume doubling time (VDT), Ki-67, and rADC were conducted with the Pearson correlation coefficient. The prediction ability was evaluated by receiver operating characteristic (ROC) curves. P values <0.05 were considered statistically significant.Results
Univariable and multivariable analyses demonstrated that only rADC is an independent predictor of meningioma growth (All, p=0.001, Figure 1A). ROC curve analysis presented in Figure 1B showed that baseline rADC had good predictive power for non-growing meningioma (AUC=0.88, p=0.001), as well as slow or fast growth meningioma (AUC=0.83, p=0.03). The log-rank test for trend (p=0.003) was statistically significant, indicating that the cumulative risk of disease progression greatly increases from the high rADC to the low rADC group (Figure 2A). The overall growth status of 64 patients is shown in Figure 2B. In 20 patients with tumor growth, rADC was moderately negatively correlated (r=-0.50, p=0.02) with tumor growth rate (Figure 3A) and strongly positively correlated (r=0.63, p=0.003) with doubling time (Figure 3B), indicating that lower rADC was associated with faster tumor growth and shorter doubling time. Typical cases in different groups (non-growth, slow growth, and fast growth) are shown in Figure 4. Moreover, Ki-67 was significantly associated with rADC in 8 patients who underwent surgery (r=-0.75, p=0.03). The corresponding scatter plot is shown in Figure 5.Discussion
Our findings suggest that meningiomas with different growth patterns differ in rADC. Meningiomas with low rADC values at baseline tend to be more likely to grow than tumors with high rADC values, while lower rADC in growing meningiomas is associated with a faster growth rate and shorter tumor doubling time. To our knowledge, this is the first time that a noninvasive DWI technique has been used to directly predict the growth of meningiomas.Conclusion
In asymptomatic meningiomas, the lower rADC at baseline, the faster TGR, and the shorter VDT. DWI could be a valuable predictive imaging tool in asymptomatic meningiomas. Patients with rapidly progressive asymptomatic meningiomas close to fragile structures such as cranial nerves or blood vessels would benefit from early surgery.Acknowledgements
No acknowledgement found.References
1. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. The Lancet Oncology, 2016, 17(9) : e383-e391.
2. Farhadi F, Nikpanah M, Paschall A, et al. Clear Cell Renal Cell Carcinoma Growth Correlates with Baseline Diffusion-weighted MRI in Von Hippel-Lindau Disease. Radiology, 2020, 295(3) : E10.
3. Surov A, Gottschling S, Mawrin C, et al. Diffusion-Weighted Imaging in Meningioma: Prediction of Tumor Grade and Association with Histopathological Parameters. Translational Oncology, 2015, 8(6) : 517-523.
Figures
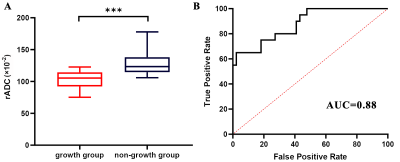
Fig. 1: Box
plot (A) of relative apparent diffusion coefficient (rADC) values and receiver
operating characteristic (ROC) curves (B) according to
growth status. Lower rADC in the growth group (median rADC value, 105.35×10-2)
compared with the non-growth group (median rADC value, 123.32×10-2;
P <0.001, Mann-Whitney U test) was observed (A). ROC curves (B) were
used to distinguish between growing and non-growing tumors. AUC = area under
the receiver operating characteristic curve.
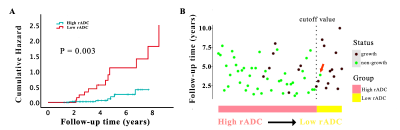
Fig. 2: Kaplan-Meier plot (A) and growth status chart (B) based on
the optimal thresholds. Figure A shows Patients with low rADC (red) had a
significantly increased cumulative risk of tumor growth compared with patients
with higher rADC (blue). Figure B shows the overall growth status of 64
patients. In the rADC-predicted tumor growth group (low rADC, yellow), tumors
grew in almost all but one patient (red arrow).
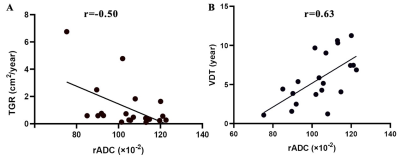
Fig. 3: Scatter plots of tumor growth rate (TGR), volume doubling time (VDT)
with relative apparent
diffusion coefficient (rADC). Scatter plots show that rADC was moderately
negatively correlated (r=-0.50, p=0.02) with tumor growth rate (A) and strongly
positively correlated (r=0.63, p=0.003) with doubling time (B).
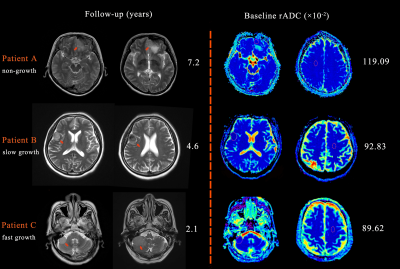
Fig. 4: Different
growth patterns of meningiomas. (A) A 51-year-old female with a left frontal
lobe meningioma of 2 cm×1.5 cm, rADC 119.09×10-2. Tumors were
similar to baseline at 7.2 years later. (B) A 59-year-old female with a lateral
frontotemporal meningioma of 2 cm×1.7 cm, rADC 92.83×10-2. Tumor
progression was recorded on MRI at the last follow-up, with a size of 2.2
cm×2.8 cm and VDT=5.4 years. (C) An 80-year-old male with a cerebellopontine
angle meningioma of 1.4 cm×2.4 cm, rADC 89.62×10-2. After 2.1 years,
a tumor of 3 cm×2.9 cm (fast growth) was recorded, with a VDT of 1.6 years.
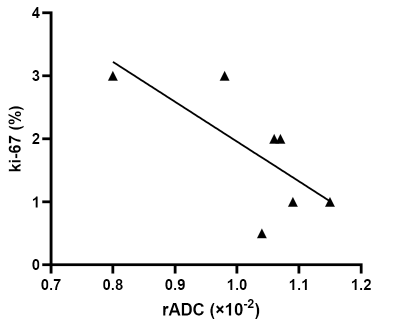
Fig. 5: Scatter plots of Ki-67 proliferation index with rADC. The Scatter plot shows that rADC was strongly
negatively correlated (r=-0.75, p=0.03) with Ki-67 proliferation index.
DOI: https://doi.org/10.58530/2023/1569